Wilson Disease Drugs Market Outlook:
Wilson Disease Drugs Market size was over USD 697.71 million in 2025 and is projected to reach USD 1.06 billion by 2035, witnessing around 4.3% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of wilson disease drugs is evaluated at USD 724.71 million.

The growth of the market can be attributed to the increasing prevalence of Wilson’s disease across the globe, especially in isolated areas. Wilson’s disease is caused by the mutation of the ATP7B gene which is passed on by parents to the child. This disease was observed to occur in 30,000 to 40,0000 people across the globe.
The advent of various organizations and associations in recent years, such as the Wilson Disease Association, an organization that endorses research and clinical investigations for Wilson disease and spreads awareness regarding the latest happening on the symptoms and treatment of Wilson disease, are observed to hike the market growth over the forecast period. Furthermore, the rising introduction of patient assistance programs which provides considerable growth opportunities to the companies for the development of novel therapeutic drugs for the treatment of Wilson disease is also anticipated to significantly propel the growth of the global Wilson disease drugs market during the forecast period.
Key Wilson Disease Drugs Market Insights Summary:
Regional Highlights:
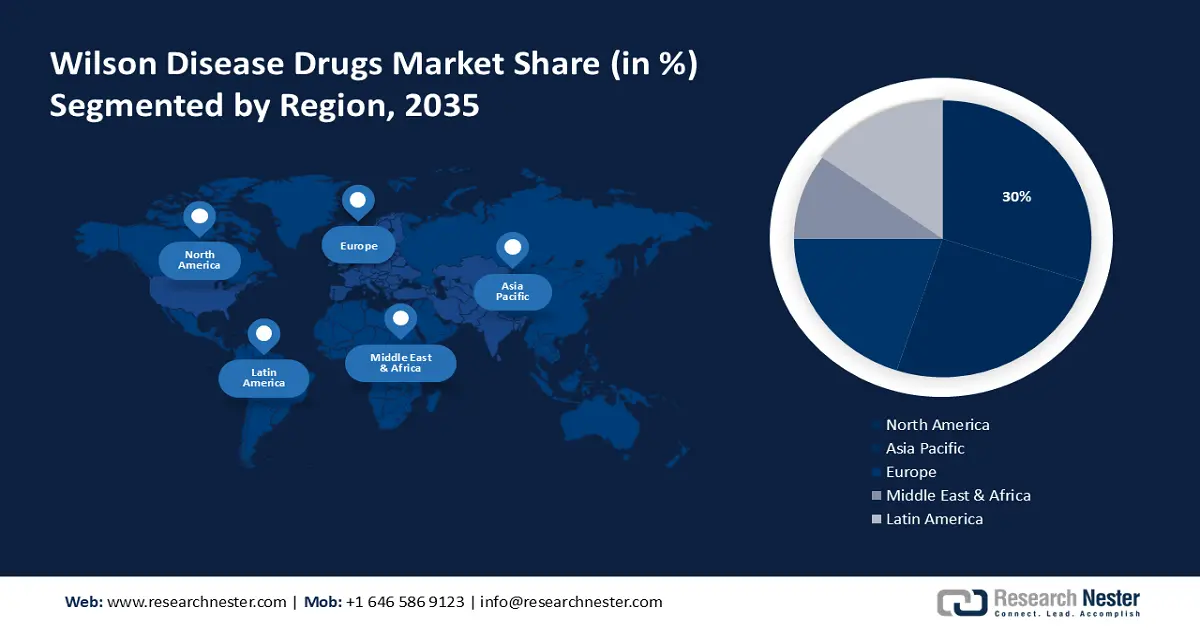
- By 2035, North America is set to capture about 30% share of the wilson disease drugs market, stemming from the presence of leading market players in the region which manufacture and distribute Wilson disease drugs.
- The Asia Pacific region is projected to hold the second-largest share by 2035, supported by rising awareness about this rare disorder and increasing healthcare expenditure in the region.
Segment Insights:
- By 2035, the neurological segment is anticipated to command the largest share of the wilson disease drugs market, propelled by the rising prevalence of neurological conditions and growing usage of neurology devices.
- The chelating agents segment is expected to secure a significant share by 2035, fueled by the escalating incidence of nervous system disorders and cancer.
Key Growth Trends:
- Growing Prevalence of Wilson’s Disease in Young Population
- Escalating Trial on Gene Therapy
Major Challenges:
- High Treatment Costs and Lack of Reimbursement Facilities
- Lack of Awareness in Low Income Countries.
Key Players: Merck & Co., Inc., Bausch Health Companies Inc., Alexion Pharmaceuticals, Inc., Nobelpharma Co., Ltd., Kodmon Holdings, Inc., Teva Pharmaceutical Industries Ltd., Tsumura & Co., VHB Medi Sciences Limited, Pfizer Inc., Lupin Limited, Vivet Therapeutics, NAVINTA LLC.
Global Wilson Disease Drugs Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 697.71 million
- 2026 Market Size: USD 724.71 million
- Projected Market Size: USD 1.06 billion by 2035
- Growth Forecasts: 4.3%
Key Regional Dynamics:
- Largest Region: North America (30% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, Japan, United Kingdom
- Emerging Countries: India, South Korea, Brazil, Australia, Saudi Arabia
Last updated on : 19 November, 2025
Wilson Disease Drugs Market - Growth Drivers and Challenges
Growth Drivers
-
Growing Prevalence of Wilson’s Disease in Young Population – Wilson’s disease generally occurs in the younger population since it is passed on to children by their parents and shows symptoms at an early age. The symptoms of Wilson’s disease include stomach aches, swelling, weariness, and others. For instance, the most common age at which symptoms of this disease appears is 12 and 13.
- Escalating Trial on Gene Therapy – gene therapy is highly advantageous in treating Wilson’s disease since it transits the working ATP7B gene into cells that create working transporter proteins. For instance, around 90% of the trial of gene therapy were noticed to be in their early phases while 70% of the were conducted in 2018 in the United States.
- Increasing Health Spending across the Globe – based on the current expenditure data, global health spending has increased over the past 20 years, doubling in real terms to hit USD 8.5 trillion in 2019 and 9.8% of GDP, up from 8.5 percent in 2000.
- Growing R&D Expenditure for Clinical Trials – for instance, in 2021, more than 400,000 studies were noticed to be conducted in 200 countries across the globe.
Challenges
-
High Treatment Costs and Lack of Reimbursement Facilities - Despite the presence of improved healthcare infrastructure, there is a high cost associated with these drugs which are used for the treatment of Wilson’s disease. In addition to this, the poor reimbursement policies in developing countries are estimated to hinder the growth of the Wilson’s disease drugs market in the future.
-
Lack of Awareness in Low-Income Countries.
-
Requirement for Higher Initial Investments
Wilson Disease Drugs Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
4.3% |
|
Base Year Market Size (2025) |
USD 697.71 million |
|
Forecast Year Market Size (2035) |
USD 1.06 billion |
|
Regional Scope |
|
Wilson Disease Drugs Market Segmentation:
Indication Segment Analysis
The global Wilson disease drugs market is segmented and analyzed for demand and supply by indication into hepatic, neurological, ophthalmic, and psychiatric. Out of these types of indications, the neurological segment is estimated to gain the largest market share in the year 2035. Wilson’s disease includes a higher number of neurological indications such as dysarthria (difficulty in speaking), dysphagia (difficulty in swallowing), tremors, poor coordination, spasticity, involuntary movements, muscle rigidity, dystonic posture, and others. Apart from that, the surge in neurological conditions and usage of neurology devices across the globe has also spurred the demand for medical drugs to cure or manage these diseases which are also expected to propel the growth of the segment over the upcoming decades. For instance, based on the statistics released by Pan American Health Organization (PAHO), it was stated that in 2019, nearly 533,172 people died owing to neurological conditions across the globe, out of which, 320,043 were women while 213,129 were men.
Drug Type Segment Analysis
The global Wilson disease drugs market is also segmented and analyzed for demand and supply by drug type into chelating agents, zinc, tetrathiomolybdate, and others. Amongst these segments, the chelating agents’ segment is expected to garner a significant share in the year 2035. Chelating agents include trientine, penicillamine, and dimercaprol which are helpful in reducing the levels of copper in blood and tissues when administered for a prolonged time period. These agents are further helpful in preventing the gathering of copper and injuries from Wilson disease. Chelating agents are developed to flush toxic metals out of the body. Toxic metals are known to cause immune system dysfunction, nervous systems disorders, cancer, birth defects, skin lesions, vascular damage, and many more. A surge in the nervous system disorder and cancer can be observed across the globe which is expected to hike the segment growth over the forecast period. As of 2020, nearly 10 million people lost their lives worldwide.
Our in-depth analysis of the global market includes the following segments:
|
By Indication |
|
|
By Drug Type |
|
|
By End-User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Wilson Disease Drugs Market - Regional Analysis
North American Market Insights
North America industry is poised to dominate majority revenue share of 30% by 2035, The growth of the market can be attributed majorly to the presence of leading market players in the region which manufacture and distribute Wilson disease drugs. Moreover, support from the government in the implementation of advanced healthcare technologies, coupled with the rise in the number of patients with hereditary disorders and Wilson’s disease in the region is anticipated to drive the demand for Wilson disease drugs in the region over the forecast period. For instance, it was estimated that in the United States, in every 30,000 individuals, one of them suffers from Wilson’s disease. Additionally, the surge in the geriatric population in this region and growing awareness among people of Wilson’s disease is also anticipated to expand the market size over the forecast period.
APAC Market Insights
The Asia Pacific Wilson disease drugs market, amongst the market in all the other regions, is projected to hold the second largest share during the forecast period. The growth of the market is backed by a rise in awareness about this rare disorder along with the increase in healthcare expenditure in the region during the forecast period. The developing economies in the region, especially the countries such as China, India, and Japan, are further anticipated to provide significant opportunities for the manufacturing of Wilson disease drugs, and in turn, drive the growth of the market in the region. In Asia Pacific, many key manufacturers are observed to invest a significant amount of money in R&D activities to find new drugs. Hence, all these factors are anticipated to influence the market growth positively over the forecast period.
Wilson Disease Drugs Market Players:
- Merck & Co., Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Bausch Health Companies Inc.
- Alexion Pharmaceuticals, Inc.
- Nobelpharma Co., Ltd.
- Kodmon Holdings, Inc.
- Teva Pharmaceutical Industries Ltd.
- Tsumura & Co.
- VHB Medi Sciences Limited
- Pfizer Inc.
- Lupin Limited
- Vivet Therapeutics
- NAVINTA LLC
Recent Developments
-
Alexion Pharmaceuticals, Inc. made an announcement regarding the acquisition of LogicBio Therapeutic, Inc. The acquisition took place to accelerate the growth of Alexion Pharmaceuticals in developing genomic medicines using the unique technology of LogicBio.
-
Kadmon Holdings, Inc. announced that its generic Trientine Hydrochloride Capsules USP, 250 mg, which is a chelating agent used for the treatment of Wilson’s disease in patients who are intolerant of penicillamine, has been approved by the U.S. Food and Drug Administration (FDA).
- Report ID: 2863
- Published Date: Nov 19, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Wilson Disease Drugs Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




