Dravet Syndrome Market Outlook:
Dravet Syndrome Market size was over USD 590.48 million in 2025 and is anticipated to cross USD 1.42 billion by 2035, witnessing more than 9.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of dravet syndrome is assessed at USD 639.37 million.
The reason behind the growth is impelled by the increasing child population. Moreover, Dravet syndrome is an uncommon kind of epilepsy that starts in infancy or early childhood and can lead to frequent, protracted seizures, which may require treatment including medication or other therapies to decrease the severity and amount of seizures.According to estimates, the number of children worldwide is projected to be over 1 billion in 2100.
The growing research & development are believed to fuel the dravet syndrome treatment market. For instance, in 2020 STK-001 an anti-sense oligonucleotide was administered, to the first patient with Dravet syndrome as part of a clinical trial to increase the expression of SCN1A in the brain.
Key Dravet Syndrome Treatment Market Insights Summary:
Regional Highlights:
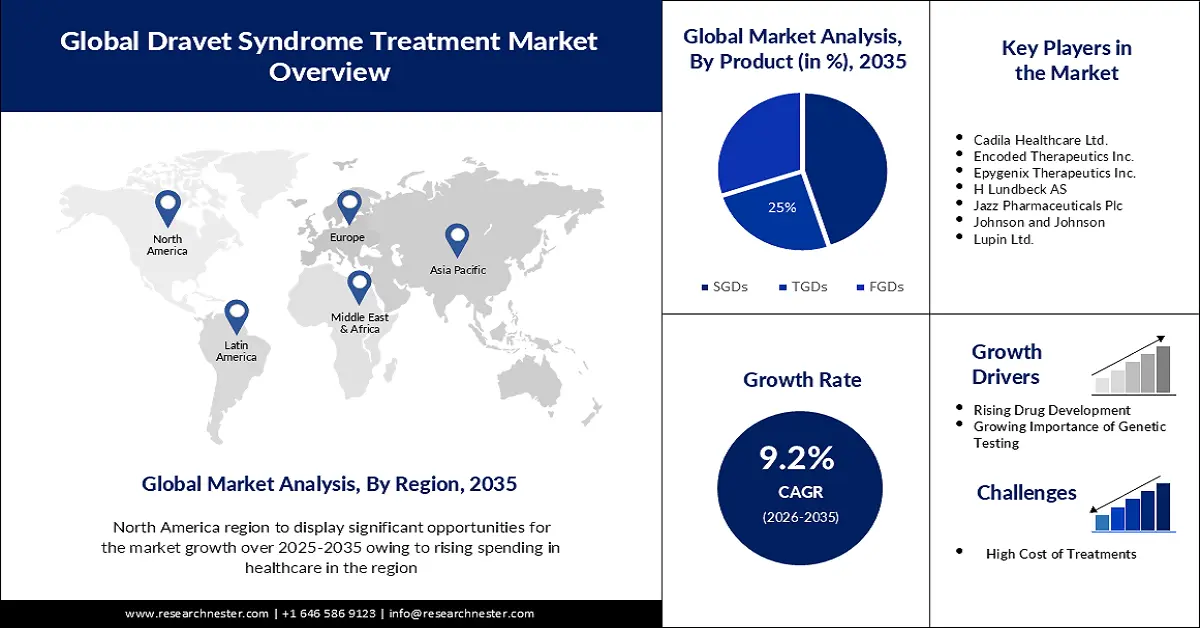
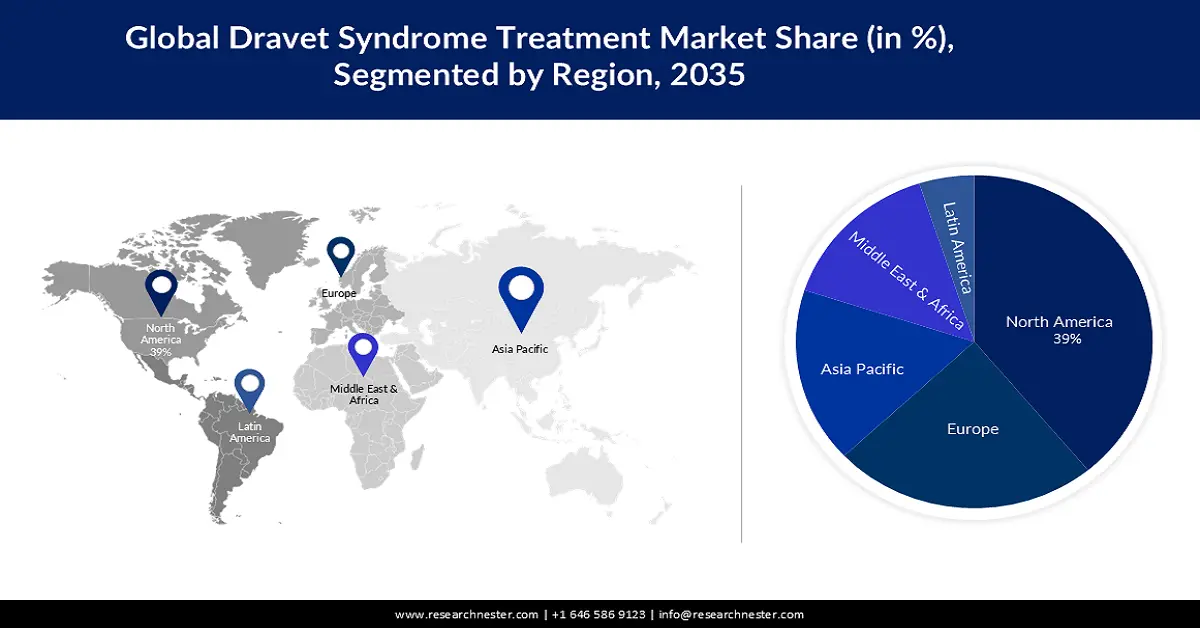
- The North America dravet syndrome market is expected to capture 39% share by 2035, driven by rising healthcare spending and R&D for Dravet syndrome treatments.
- The Europe market will secure the second largest share by 2035, driven by drug development activities expanding treatment options for Dravet syndrome.
Segment Insights:
- The sgds segment in the dravet syndrome market is anticipated to capture a 45% share by 2035, driven by the availability of stiripentol proven to treat Dravet-related seizures.
Key Growth Trends:
- Rising Drug Development
- Growing Importance of Genetic Testing
Major Challenges:
- High Cost of Treatments
- Complexity Associated With Meeting Regulatory Requirements for the Approval of Rare Disease Treatment
Key Players: Cadila Healthcare Ltd., Encoded Therapeutics Inc., Epygenix Therapeutics Inc., H Lundbeck AS, Jazz Pharmaceuticals Plc, Johnson and Johnson, Lupin Ltd., PTC Therapeutics Inc., Stoke Therapeutics Inc.
Global Dravet Syndrome Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 590.48 million
- 2026 Market Size: USD 639.37 million
- Projected Market Size: USD 1.42 billion by 2035
- Growth Forecasts: 9.2% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (39% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, United Kingdom, France
- Emerging Countries: China, India, Japan, South Korea, Brazil
Last updated on : 11 September, 2025
Dravet Syndrome Market Growth Drivers and Challenges:
Growth Drivers
- Rising Drug Development – The FDA may collaborate with businesses to develop medications for Dravet syndrome, which is expected to drive market growth. According to recent estimates, the typical R&D expenditure for a new medicine ranges from more than USD 1 billion.
- Growing Importance of Genetic Testing- Genetic testing is a straightforward blood test that can reveal sequencing abnormalities in the SCN1A gene that can help in determining whether an individual has Dravet syndrome by analyzing the most prevalent genes linked to epilepsy.
Challenges
- High Cost of Treatments - Dravet syndrome is a rare disease that affects a small percentage of people, therefore the medicines for treating this disease are exceedingly expensive, presumably to cover the cost of the research and development. For instance, more than a 20% rise in overall expenses is linked to the absence of treatment for a rare condition.
- Complexity Associated With Meeting Regulatory Requirements for the Approval of Rare Disease Treatment
- Lack of Awareness among Doctors and Patients Owing to Rarity of Dravet Syndrome
Dravet Syndrome Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
9.2% |
|
Base Year Market Size (2025) |
USD 590.48 million |
|
Forecast Year Market Size (2035) |
USD 1.42 billion |
|
Regional Scope |
|
Dravet Syndrome Market Segmentation:
Product Segment Analysis
The SGDs segment is estimated to account for 45% share of the dravet syndrome treatment market in the coming years. The only SGDs that are currently available for the treatment of DS are stiripentol and topiramate out of which the only medication that has been proven to adequately treat Dravet-related seizures is stiripentol which is available in the form of hard capsules or a powder for oral suspension. Besides this, it can be used as an orphan medication in the supplementary management of childhood epilepsy syndrome.
End-User Segment Analysis
Dravet syndrome treatment market from the hospital pharmacies segment is set to garner a notable share shortly. Drugs, such as valproate, clobazam, stiripentol, cannabidiol, or fenfluramine, are used to treat patients with Dravet syndrome which are provided by hospital pharmacies as it has the required skills for pricing, dispensing, and the availability of skilled hospital pharmacists to manage these medications.
Treatment Type Segment Analysis
The seizure medications segment in the dravet syndrome treatment market is set to garner a significant share during the forecast timeframe. Seizure medications work to halt or prevent seizures by functioning in a variety of ways. For instance, more than 60% of epilepsy sufferers can control their seizures with medication. Moreover, anti-seizure drugs function by lessening the aberrant electrical activity in the brain and help in managing the type of pain brought on by damaged nerves.
Diagnosis Type Segment Analysis
The magnetic resonance imaging segment in the dravet syndrome treatment market is set to garner a significant share by the year 2035. Dravet syndrome (DS) and other neurological conditions may be distinguished using (MRI) since it creates extremely accurate images of a child's brain using a magnetic field and radio waves.
Our in-depth analysis of the global market includes the following segments:
|
Seizures Type |
|
|
Diagnosis Type |
|
|
End-User |
|
|
Treatment Type |
|
|
Product |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Dravet Syndrome Market Regional Analysis:
North American Market Insights
Dravet syndrome treatment market in North America industry is predicted to dominate majority revenue share of 39% by 2035 impelled by the rising spending in healthcare. This has led to an increase in research & development of more efficient and effective treatments for Dravet syndrome in the region, which would also improve access to the treatment of the disease in the region. According to estimates, in 2021, the United States spent over 2% more on health care, amounting to roughly USD 4 trillion.
European Market Insights
The Europe dravet syndrome Treatment market is estimated to be the second largest, during the forecast timeframe led by growing development of new drugs. For instance, Europe serves as the world's center for medication development, as over 72 novel opioids have been discovered on the European drug market between 2009 and 2022. This has led to an expansion in treatment options for Dravet syndrome in the region.
Dravet Syndrome Market Players:
- AbbVie Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Cadila Healthcare Ltd.
- Encoded Therapeutics Inc.
- Epygenix Therapeutics Inc.
- H Lundbeck AS
- Jazz Pharmaceuticals Plc
- Johnson and Johnson
- Lupin Ltd.
- PTC Therapeutics Inc.
- Stoke Therapeutics Inc.
- Supernus Pharmaceuticals Inc.
- Sun Pharmaceutical Industries Ltd.
Recent Developments
- Cadila Healthcare Ltd. an innovative, global pharmaceutical company received approval from the USFDA for a seizure treatment drug, vigabatrin tablets in the strength of 500 mg, which is capable of reducing the frequency of seizures in both adults and kids who haven't been able to manage their seizures with existing treatments by preventing the breakdown of GABA, a naturally occurring tranquilizer, in the brain.
- Encoded Therapeutics Inc. received over USD 130 million to fund the initial human clinical studies of ETX101, which was given classifications as an orphan drug and a rare pediatric condition by the American Food and Drug Administration (FDA). Furthermore, ETX101 was created to raise NaV1.1-alpha levels to prepare it for the clinic and beyond, and to attract some of the most knowledgeable senior leaders in gene therapy.
- Report ID: 5280
- Published Date: Sep 11, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Dravet Syndrome Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




