Angiosarcoma Treatment Market Outlook:
Angiosarcoma Treatment Market size was over USD 292.76 million in 2025 and is projected to reach USD 500.08 million by 2035, growing at around 5.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of angiosarcoma treatment is evaluated at USD 307.25 million.

The elderly population has a greater cancer risk, thus resulting in the demand for angiosarcoma treatment. Over 703 million people over the age of 65 live in the world today, making up 9.1% of all people, according to the Union for International Cancer Control (UICC). By 2050, it is predicted that this percentage would increase to 15.9%. According to UICC, the total cancer-related death rate for those aged 70 and above is expected to be 65,17,865 by 2030. Angiosarcomas Treatment Industry would expand over the forecast period as a result of an increasing incidence and high mortality rate of cancer-related diseases in the senior population.
The increased use of sophisticated cancer therapeutic alternatives and the rising demand for targeted drugs and immunotherapy as disease treatments both contribute to the market's rapid expansion. The development of bioinformatic tools increasing the drug development process as well as the introduction of emerging therapeutic classes, such as monoclonal antibodies and histone deacetylase (HDAC) inhibitors, have a significant impact on the market.
Key Angiosarcoma Treatment Market Insights Summary:
Regional Highlights:
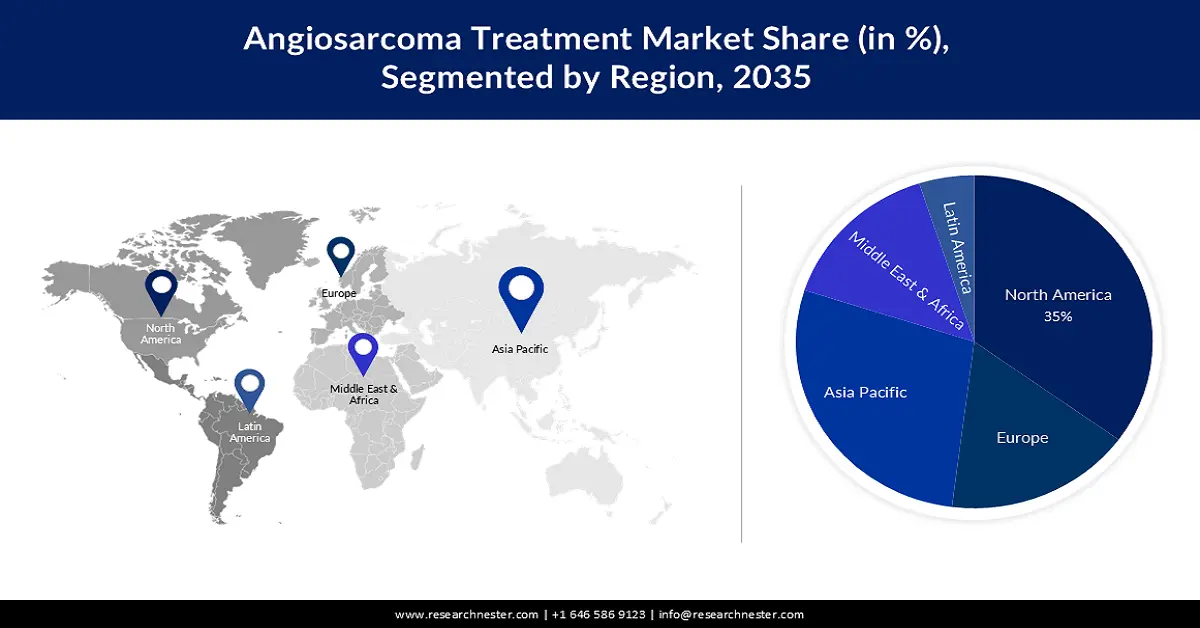
- By 2035, North America is anticipated to command a 35% share of the angiosarcoma treatment market, attributed to advancing medical infrastructure and supportive government R&D policies.
- The Asia Pacific region is projected to witness substantial expansion through 2035, stemming from the rising elderly population with higher cancer susceptibility.
Segment Insights:
- The surgery segment is expected to secure the largest revenue share by 2035 in the angiosarcoma treatment market, propelled by the escalating global demand for cancer surgeries.
- The ambulatory care center segment is poised to grow rapidly through 2035, supported by increasing preference for outpatient cancer treatment facilities.
Key Growth Trends:
- Increasing Research and Development for Angiosarcoma Treatment
- Rising Technological Advancement for Angiosarcoma Treatment
Major Challenges:
- Complexities Associated with Lymphangiosarcoma
- High Cost of Treatment is Assumed to Hamper the Market Expansion in the Upcoming Time Period.
Key Players: Bayer AG, GlaxoSmithKline plc., Sanofi S.A., Amgen Inc., Merck & Co. Inc., AbbiVie Inc., Eli Lilly and Company, Bausch Health.
Global Angiosarcoma Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 292.76 million
- 2026 Market Size: USD 307.25 million
- Projected Market Size: USD 500.08 million by 2035
- Growth Forecasts: 5.5%
Key Regional Dynamics:
- Largest Region: North America (35% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, Japan, United Kingdom
- Emerging Countries: India, Brazil, South Korea, Australia, Mexico
Last updated on : 25 November, 2025
Angiosarcoma Treatment Market - Growth Drivers and Challenges
Growth Drivers
- Increasing Research and Development for Angiosarcoma Treatment - Due to numerous recent studies and advancements, the angiosarcoma treatment market has seen substantial expansion. Clinical and radiological data, along with medical history, are typically used to make the diagnosis of angiosarcoma. Additionally, the introduction of new therapies for angiosarcoma aids in quickening market expansion. For example, a November 2022 article titled Cutaneous angiosarcoma treated with taxane-based radiation therapy mentions the significance of taxane-derived agents like paclitaxel and docetaxel as primary chemotherapy for the treatment of cutaneous angiosarcoma. In addition, radiation therapy with taxanes significantly improved overall survival relative to surgery or radiotherapy and had a high response rate.
- Rising Technological Advancement for Angiosarcoma Treatment - The inner membrane of blood arteries and lymph vessels is where angiosarcoma occurs, and significant developments from top firms are fostering angiosarcoma treatment market expansion. With cutaneous angiosarcoma accounting for about 57.21% of cases, the skin is the area most commonly afflicted. These successful developments contribute to the development of novel therapeutic approaches and the enhancement of care for the vast majority of angiosarcoma patients. For instance, a Springer Journal paper from April 2023 claims that topological immunological pathways of Asian head and neck angiosarcoma are revealed by spatial transcriptomics. It entails revealing the geometric tumor immunological landscape of angiosarcoma using spatial transcriptome profiling utilizing the 10x Genomics Visium platform on typical cases distinguished by the existence or absence of UV signals and HHV-7.
Challenges
- Complexities Associated with Lymphangiosarcoma - Complications of lymphoma include cellulitis, musculoskeletal pain, psychological distress, and increased risk of malignancy. Cellulitis often occurs with oral antibiotics, and cellulitis is a bacterial infection of the dermis and subcutaneous tissue under the skin and is the most common complication associated with lymphoma. Thus, due to the above factors, the market is expected to slow down during the forecast period.
- High Cost of Treatment is Assumed to Hamper the Market Expansion in the Upcoming Time Period.
- Lack of Effective Treatments is Set to Restrict the Market Growth in the Forecast Timeframe.
Angiosarcoma Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
5.5% |
|
Base Year Market Size (2025) |
USD 292.76 million |
|
Forecast Year Market Size (2035) |
USD 500.08 million |
|
Regional Scope |
|
Angiosarcoma Treatment Market Segmentation:
Treatment Segment Analysis
Angiosarcoma treatment market from the surgery segment is anticipated to account for the largest revenue share during the forecast period. This is because the need for biopsies to study various types of cancer is increasing. It also helps in proper diagnosis and treatment and plays an important role in various types of cancer services. According to cancer statistics, in 2021, the American Cancer Society performed more than 1 million biopsies in the United States. Due to the prevalence of cancer globally, increased demand for cancer surgery has been reported in recent years. According to a study published in January 2021, indications for cancer surgery will increase by 5 million interventions (52%) from 2018 (90,65,000) to 2040 (1.38 ,21,000). Such increasing demand for cancer surgery is driving the growth in angiosarcoma treatment market share.
End-Users Segment Analysis
Based on end user, the ambulatory care center segment is poised to be the fastest growing segment in the angiosarcoma treatment market. This may be due to the characteristics of ambulatory centers that provide patients with chemotherapy regimens in an inpatient setting and supportive care provided in an outpatient setting. There, instead of spending the night in a hospital bed, patients stay in a nearby hotel or in their homes while receiving daily treatment and supportive care in an outpatient care facility. Because of these facilities, most people prefer outpatient care centers to long-term hospital stays. The growing popularity of outpatient centers to treat various cancers is driving the growth of angiosarcoma treatments in the projected time period.
Our in-depth analysis of the global angiosarcoma treatment market includes the following segments:
|
Treatment |
|
|
Location of Instigation |
|
|
End-Users |
|
|
Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Angiosarcoma Treatment Market - Regional Analysis
North America Market Insights
The North America industry is poised to dominate majority revenue share of 35% by 2035. This is due to the development of medical infrastructure and government policies that favor the research and development of new drugs and treatments. According to the National Cancer Institute, the cancer research budget increased by 99.3 million (1.6%) from 2020 to 2021. Additionally, cases of angiosarcomas as well as lymphedema and syncope are increasing. increased in this area. In the United States, one case of angiosarcoma is diagnosed each year in every million people. About 1 to 2% of all sarcomas are angiosarcomas.
APAC Market Insights
The angiosarcoma treatment market in Asia Pacific region is expected to grow substantially during the forecast period. The growth of this market can be ascribed on the back of an increasing number of old age population found in this region. Due to weakening body functions and less immunity the old population in the region majorly suffers from cancer as compared to the middle or young adults which is predicted to raise the market growth of the angiosarcoma treatment market in the projected time period.
Angiosarcoma Treatment Market Players:
- F. Hoffmann La Roche Ltd.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Novartis AG
- Bayer AG
- GlaxoSmithKline plc.
- Sanofi S.A.
- Amgen Inc.
- Merck & Co. Inc.
- AbbiVie Inc.
- Eli Lilly and Company
- Bausch Health
Recent Developments
- Novartis announced that the U.S. Food and Drug Administration (FDA) had granted commercial authorization for a multi-product gene therapy manufacturing facility in Durham, North Carolina. This approval allows the 170,000 square foot state-of-the-art facility to manufacture, test and commercialize Zolgensma, as well as produce gene therapy products for current and future clinical trials.
- Amgen announced the completion of its acquisition of Teneobio, Inc. was previously announced with a cash payment of $900 million. The acquisition includes Teneobio's proprietary specific and multispecific antibody technologies, which complement Amgen's existing antibody capabilities and the BiTE platform, and will enable significant acceleration and efficiency in the discovery and development of new molecules.
- Report ID: 5327
- Published Date: Nov 25, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Angiosarcoma Treatment Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




