Hereditary Angioedema Treatment Market Outlook:
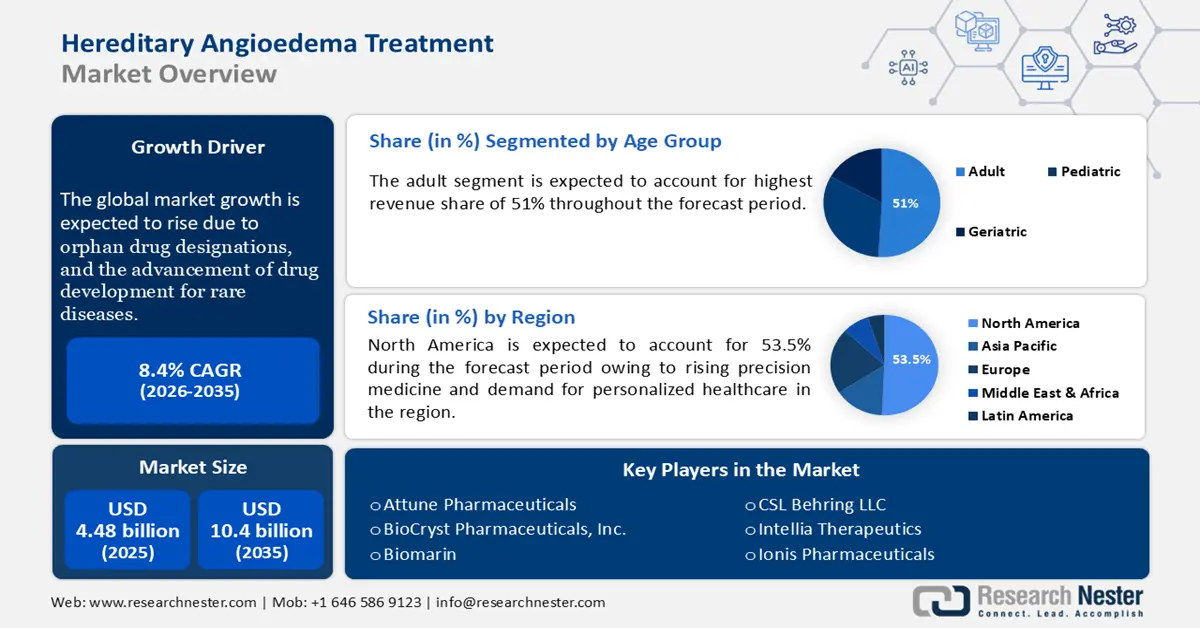
Hereditary Angioedema Treatment Market size was valued at USD 4.48 billion in 2025 and is expected to reach USD 10.04 billion by 2035, expanding at around 8.4% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of hereditary angioedema treatment is evaluated at USD 4.82 billion.
The hereditary angioedema treatment market is expanding rapidly, fueled by the advancements in treatment options, improved diagnosis, and rising awareness of the disease. Furthermore, growing accessibility to novel biologics, gene therapies, and preventive treatments aimed at reducing the frequency and severity of HAE attacks is also boosting market growth. As per the Rare Disease Advisor, hereditary angioedema affects 1 in every 50,000 people globally.
The rise of patient advocacy groups, orphan drug designations, and the promotion of drug development for rare diseases are driving the hereditary angioedema treatment market expansion. On-going clinical trials focused on long-term curative solutions, and addressing the rising therapy demands are two factors majorly explored by the market players. For instance, in February 2024, KalVista Pharmaceuticals, Inc. announced positive results from the phase 3 KONFIDENT clinical trial. KONFIDENT demonstrated statistically and clinically significant efficacy of sebetralstat as an oral on-demand therapy for HAE. Personalized medicine approaches and advancements in genomic research are expected to further shape the market’s future.
Key Hereditary Angioedema Treatment Market Insights Summary:
Regional Highlights:
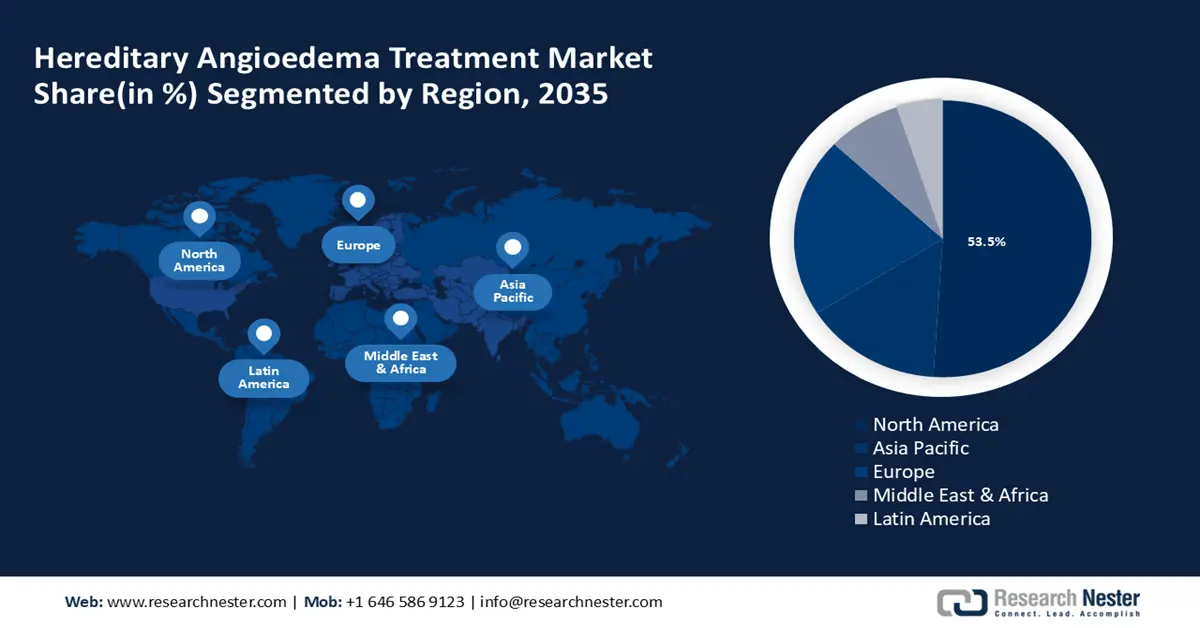
- North America leads the Hereditary Angioedema Treatment market with a 53.5% share, propelled by the rise in precision medicine and personalized healthcare demand, fostering innovation through 2026–2035.
Segment Insights:
- The Hospital Pharmacies segment is anticipated to experience considerable growth from 2026-2035, propelled by the need for specialized care during severe HAE attacks requiring immediate intervention.
- The Adult segment of the Hereditary Angioedema Treatment Market is projected to hold over 51% share by 2035, driven by higher prevalence and severity of attacks increasing treatment demand.
Key Growth Trends:
- Rising case of HAE
- Technological advances in diagnostics and new drug developments
Major Challenges:
- Stringent regulations on new HAE therapies
- High cost of treatment
- Key Players: BioCryst Pharmaceuticals, Inc., Biomarin, CSL Behring LLC.
Global Hereditary Angioedema Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 4.48 billion
- 2026 Market Size: USD 4.82 billion
- Projected Market Size: USD 10.04 billion by 2035
- Growth Forecasts: 8.4% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (53.5% Share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, Germany, Japan, United Kingdom, France
- Emerging Countries: China, India, Japan, South Korea, Singapore
Last updated on : 14 August, 2025
Hereditary Angioedema Treatment Market Growth Drivers and Challenges:
Growth Drivers
- Rising case of HAE: An article published by the Rare Disease Advisor in June 2022, states that HAE episodes lead to 15,000 to 30,000 annual emergency department admissions, in the U.S. As awareness of the condition increases, more individuals are being diagnosed, leading to a greater demand for effective treatments. The rising cases are also attributed to an improved understanding of the genetic factors of the disease. This leads to early detection, and intervention, which boosts the market for therapies aimed at managing symptoms and preventing attacks. The rising patient pool is projected to boost the demand for both acute symptom management and preventive measures to reduce the attack frequency.
- Technological advances in diagnostics and new drug developments: Innovations in biotechnology, including gene editing, monoclonal antibodies, and biologics have led to the creation of more targeted therapies for managing HAE. These advances allow for treatments that not only provide symptom relief but also prevent attacks. Additionally, improved drugs formulations are making therapy more accessible for patients. In January 2024, Pharvaris announced that the U.S. FDA has lifted the clinical hold on the Investigational New Drug (IND) application for deucrictibant for the prophylactic treatment of HAE attacks. As new drugs continue to emerge, alongside technological advancements, the HAE market is poised for significant growth, with better-targeted treatments.
Challenges
- Stringent regulations on new HAE therapies: This poses a significant challenge for the market as the approval process for novel treatments is often lengthy and complex owing to the extensive need for clinical trials to ensure safety and efficacy. Regulatory authorities impose rigorous standards, which can delay the introduction of new therapies, limiting the availability of treatment options for HAE patients.
- High cost of treatment: A major challenge faced by the hereditary angioedema treatment market is the rising cost of advanced treatment for HAE. Owing to the specialized nature of the medications and the small patient population target, many current therapies are severely expensive. This creates financial barriers for patients and healthcare systems, restricting access to effective treatments, mainly in regions with limited healthcare resources.
Hereditary Angioedema Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
8.4% |
|
Base Year Market Size (2025) |
USD 4.48 billion |
|
Forecast Year Market Size (2035) |
USD 10.04 billion |
|
Regional Scope |
|
Hereditary Angioedema Treatment Market Segmentation:
Distribution Channel (Hospital Pharmacies, Drug Store & Retail Pharmacies, Online Pharmacies)
By distribution channel, hospital pharmacies in hereditary angioedema treatment market are anticipated to grow at a considerable CAGR during the forecast period. The need for specialized care in managing severe and acute HAE attacks often require immediate medical intervention and the administration of advanced therapies including C1 inhibitors. Hospitals are equipped with the necessary resources to handle these emergencies, including diagnostic tools and skilled healthcare professionals trained in rare disease management. The rising prevalence of HAE has increased the number of hospital visits for both emergency treatment and regular monitoring.
Increasing number of clinical trials and development of novel therapies conducted in hospital settings is also boosting the segment’s growth. Hospitals are centers for innovatie treatments such as gene therapies, which are initially introduced through clinical trial programs. Furthermore, adoption of more advanced therapeutic protocols in hospitals, such as precision therapies, and personalized medicine, is projected to boost enhances patient outcomes. Development of specialized HAE treatment centers within hospitals is also likely to drive the segment’s growth during the forecast period.
Age Group (Pediatric, Adult, Geriatric)
Adult segment is projected to account for more than 51% hereditary angioedema treatment market share by the end of 2035. The segment is majorly driven by factors including late onset or delayed diagnosis of HAE where symptoms are more accurately identified during adulthood. Additionally, adults tend to experience more frequent and severe attacks, increasing the demand for preventive and acute treatments. Lifestyle factors such as stress, hormonal changes, and work-related pressures also trigger HAE attacks, further contributing to the segment’s growth.
Tailored treatment plans owing to personalized medicines advancements based on genetic and symptomatic profiles, ensure better disease management for adult patients. Furthermore, development of long-term prophylactic treatments and greater use of self-administration therapies are anticipated in the market. This will empower adult patients to manage their conditions more independently is projected to further fuel the market growth during the forecast period.
Our in-depth analysis of the market includes the following segments:
|
Treatment Type |
|
|
Route of Administration |
|
|
Age Group |
|
|
Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Hereditary Angioedema Treatment Market Regional Analysis:
North America Market Analysis
North America industry is anticipated to account for largest revenue share of 53.5% by 2035. The market encompasses therapies designed to treat conditions inherited through genetic mutations. This includes gene therapies, enzyme replacement therapies, and gene editing technologies. The rise in precision medicine and personalized healthcare has further fueled the demand for these treatments in the region. Moreover, the presence of prominent players and their R&D and product developments are also significantly boosting the region’s market growth. For instance, in March 2024, Astria Therapeutics announced positive initial proof-of-concept outcomes from the ALPHA-STAR Phase 1b/2 clinical trial evaluating STAR-0215. It is a monoclonal antibody inhibitor of plasma kallikrein, in hereditary angioedema (HAE) patients.
The National Library of Medicine conducted an internet-based survey among the US physicians. The survey concluded that about 1,230 to 1,331 people in the U.S. suffered from HAE-nl-C1INH between May 2019 and April 2020. Owing to the rising number of HAE cases since 2020, the U.S. market is witnessing significant growth, which is further driven by improved diagnosis of the rare genetic disorder. Prominent players in the market are focusing on developing novel biologics to provide long-term solutions. The market is also supported by favorable reimbursement policies and ongoing clinical research.
With better diagnostic practices and improved access to advanced treatments in Canada the HAE market is showcasing steady growth in the country. A robust healthcare system and ongoing government support for rare disease treatment are also boosting the market. Companies in the country are increasingly investing in developing advanced devices. In November 2021, BioCryst Pharmaceutical, Inc., Royalty Pharma plc, and OMERS Capital Markets announced the addition of USD 350 million in new funding for BioCryst. The added funds are aimed to enable further advancement of BCX9930 and also support the global launch of ORLADEYO.
Europe Market Statistics
Increased awareness of the condition, and improved diagnostic rates, in addition to the development of novel therapies by the prominent players in the region, are driving the Europe market steadily. Well-established healthcare facilities and regulatory frameworks encourage the development and adoption of orphan drugs for rare diseases including HAE. Germany, Italy, and France are leading contributors to the region’s market growth. Prominent players are also presenting a great number of developments in the region. For instance, in December 2023, Otsuka Pharmaceuticals Co., Inc., entered a license agreement where Otsuka acquired exclusive marketing rights to Ionis HAE drug candidate donidalorsen in Europe. Organizations including European Medicines Agency, and rare diseases advocacy groups are playing a vital role in facilitating the approval and reimbursement of HAE therapies.
Germany healthcare system facilitates early diagnosis and treatment of rare diseases, including HAE. As per the National Library of Medicine, in January 2024, there were approximately 1,700 patients suffering from HAE in Germany. The country’s regulatory bodies and health insurance systems also play a significant role in ensuring patient access to these advanced treatments. The presence of prominent players in the hereditary angioedema treatment market is also boosting the growth significantly.
France focuses on patient-centric care and the availability of novel treatments, in addition to its comprehensive coverage and public support for rare diseases. These factors are driving the country’s market successfully. Organizations such as the French National Rare Disease Plan support research, diagnosis, and patient care for conditions including HEA. Furthermore, the country’s extensive network of specialist centers for rare diseases ensures that the patients receive appropriate care and management. Hence, the country’s market is projected to witness considerable growth in the upcoming years.
Key Hereditary Angioedema Treatment Market Players:
- Attune Pharmaceuticals
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- BioCryst Pharmaceuticals, Inc.
- Biomarin
- CSL Behring LLC
- Intellia Therapeutics
- Ionis Pharmaceuticals
- KalVista Pharmaceuticals, Inc.
- Pharming Healthcare, Inc.
- Pharvaris
The hereditary angioedema treatment market is dominated is dominated by several key companies, significantly shaping the landscape of innovative treatments. These companies focus on both acute and prophylactic treatments. In September 2021, Cycle Pharmaceuticals announced the launch of SAJAZIR Injection, a new treatment option for patients affected by Hereditary Angioedema. The treatment has been approved by the US Food and Drug Administration (FDA). Competitive dynamics in the hereditary angioedema treatment market have intensified in the last few years. Companies are seeking to differentiate their products through improved efficacy, safety, and ease of use. Some of these prominent companies are:
Recent Developments
- In January 2024, Ionis Pharmaceuticals, Inc. announced positive results for the Phase 3 OASIS-HAE study of donidalorsen in people with hereditary angioedema
- In March 2023, Intellia Therapeutics received FDA clearance for an Investigational New Drug (IND) application for NTLA-2002. It is an in vivo genome editing candidate designed to inactivate the target gene and prevent HAE attacks after a single-dose treatment.
- In November 2022, BioCryst Pharmaceuticals, Inc. received marketing authorization from the Israeli Ministry of Health for oral, once-daily ORLADEYO to prevent HAE attacks.
- In July 2021, Orchard Therapeutics and Pharming Group N.V. announced a strategic collaboration to research, develop, manufacture, and commercialize OTL-105, for the treatment of hereditary angioedema.
- Report ID: 6520
- Published Date: Aug 14, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




