Hereditary Angioedema Treatment Market Outlook:
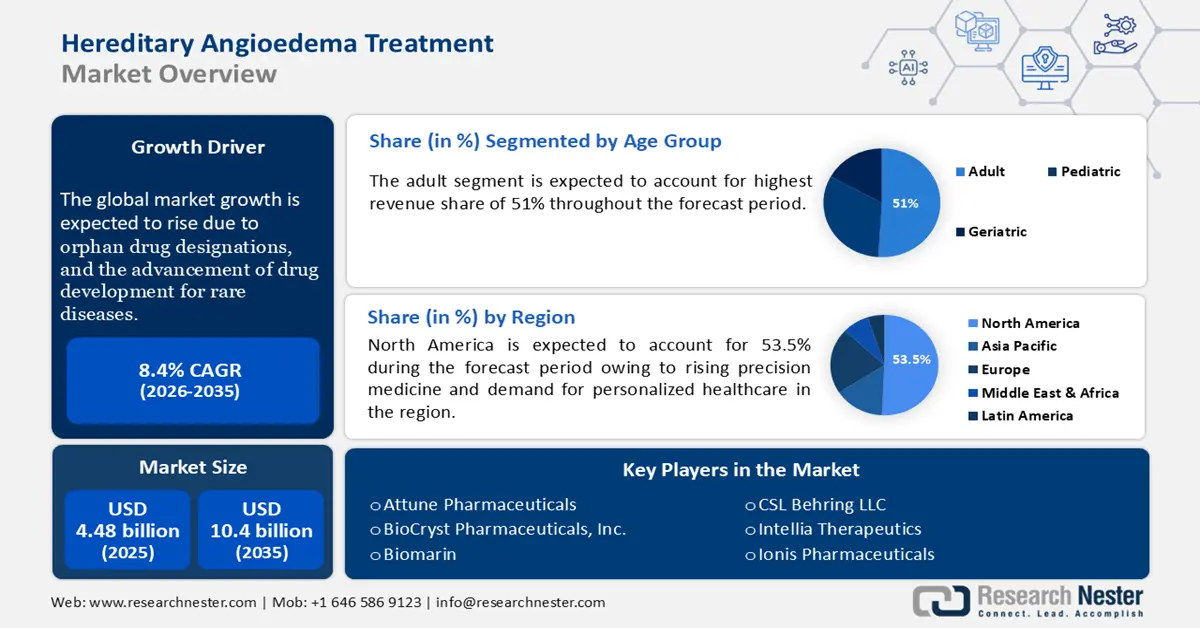
Hereditary Angioedema Treatment Market size was valued at USD 4.48 billion in 2025 and is expected to reach USD 10.04 billion by 2035, expanding at around 8.4% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of hereditary angioedema treatment is evaluated at USD 4.82 billion.
The hereditary angioedema treatment market is expanding rapidly, fueled by the advancements in treatment options, improved diagnosis, and rising awareness of the disease. Furthermore, growing accessibility to novel biologics, gene therapies, and preventive treatments aimed at reducing the frequency and severity of HAE attacks is also boosting market growth. As per the Rare Disease Advisor, hereditary angioedema affects 1 in every 50,000 people globally.
The rise of patient advocacy groups, orphan drug designations, and the promotion of drug development for rare diseases are driving the hereditary angioedema treatment market expansion. On-going clinical trials focused on long-term curative solutions, and addressing the rising therapy demands are two factors majorly explored by the market players. For instance, in February 2024, KalVista Pharmaceuticals, Inc. announced positive results from the phase 3 KONFIDENT clinical trial. KONFIDENT demonstrated statistically and clinically significant efficacy of sebetralstat as an oral on-demand therapy for HAE. Personalized medicine approaches and advancements in genomic research are expected to further shape the market’s future.