Hereditary Angioedema Treatment Market Regional Analysis:
North America Market Analysis
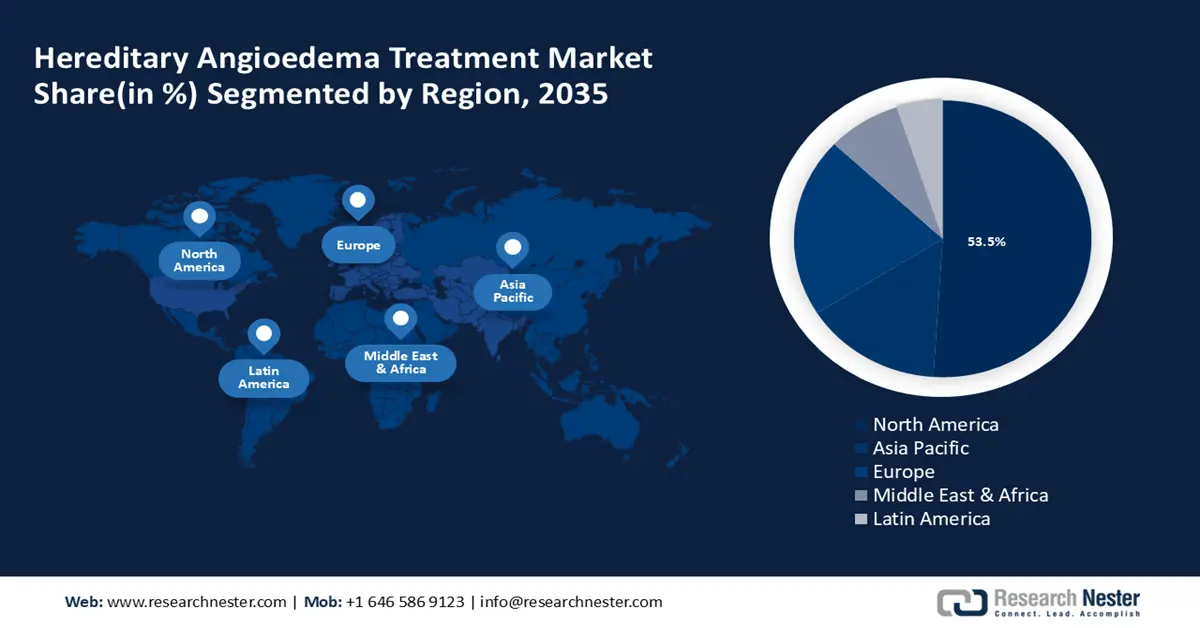
North America industry is anticipated to account for largest revenue share of 53.5% by 2035. The market encompasses therapies designed to treat conditions inherited through genetic mutations. This includes gene therapies, enzyme replacement therapies, and gene editing technologies. The rise in precision medicine and personalized healthcare has further fueled the demand for these treatments in the region. Moreover, the presence of prominent players and their R&D and product developments are also significantly boosting the region’s market growth. For instance, in March 2024, Astria Therapeutics announced positive initial proof-of-concept outcomes from the ALPHA-STAR Phase 1b/2 clinical trial evaluating STAR-0215. It is a monoclonal antibody inhibitor of plasma kallikrein, in hereditary angioedema (HAE) patients.
The National Library of Medicine conducted an internet-based survey among the US physicians. The survey concluded that about 1,230 to 1,331 people in the U.S. suffered from HAE-nl-C1INH between May 2019 and April 2020. Owing to the rising number of HAE cases since 2020, the U.S. market is witnessing significant growth, which is further driven by improved diagnosis of the rare genetic disorder. Prominent players in the market are focusing on developing novel biologics to provide long-term solutions. The market is also supported by favorable reimbursement policies and ongoing clinical research.
With better diagnostic practices and improved access to advanced treatments in Canada the HAE market is showcasing steady growth in the country. A robust healthcare system and ongoing government support for rare disease treatment are also boosting the market. Companies in the country are increasingly investing in developing advanced devices. In November 2021, BioCryst Pharmaceutical, Inc., Royalty Pharma plc, and OMERS Capital Markets announced the addition of USD 350 million in new funding for BioCryst. The added funds are aimed to enable further advancement of BCX9930 and also support the global launch of ORLADEYO.
Europe Market Statistics
Increased awareness of the condition, and improved diagnostic rates, in addition to the development of novel therapies by the prominent players in the region, are driving the Europe market steadily. Well-established healthcare facilities and regulatory frameworks encourage the development and adoption of orphan drugs for rare diseases including HAE. Germany, Italy, and France are leading contributors to the region’s market growth. Prominent players are also presenting a great number of developments in the region. For instance, in December 2023, Otsuka Pharmaceuticals Co., Inc., entered a license agreement where Otsuka acquired exclusive marketing rights to Ionis HAE drug candidate donidalorsen in Europe. Organizations including European Medicines Agency, and rare diseases advocacy groups are playing a vital role in facilitating the approval and reimbursement of HAE therapies.
Germany healthcare system facilitates early diagnosis and treatment of rare diseases, including HAE. As per the National Library of Medicine, in January 2024, there were approximately 1,700 patients suffering from HAE in Germany. The country’s regulatory bodies and health insurance systems also play a significant role in ensuring patient access to these advanced treatments. The presence of prominent players in the hereditary angioedema treatment market is also boosting the growth significantly.
France focuses on patient-centric care and the availability of novel treatments, in addition to its comprehensive coverage and public support for rare diseases. These factors are driving the country’s market successfully. Organizations such as the French National Rare Disease Plan support research, diagnosis, and patient care for conditions including HEA. Furthermore, the country’s extensive network of specialist centers for rare diseases ensures that the patients receive appropriate care and management. Hence, the country’s market is projected to witness considerable growth in the upcoming years.