Scleroderma Therapeutics Market Regional Analysis:
North America Market Forecast
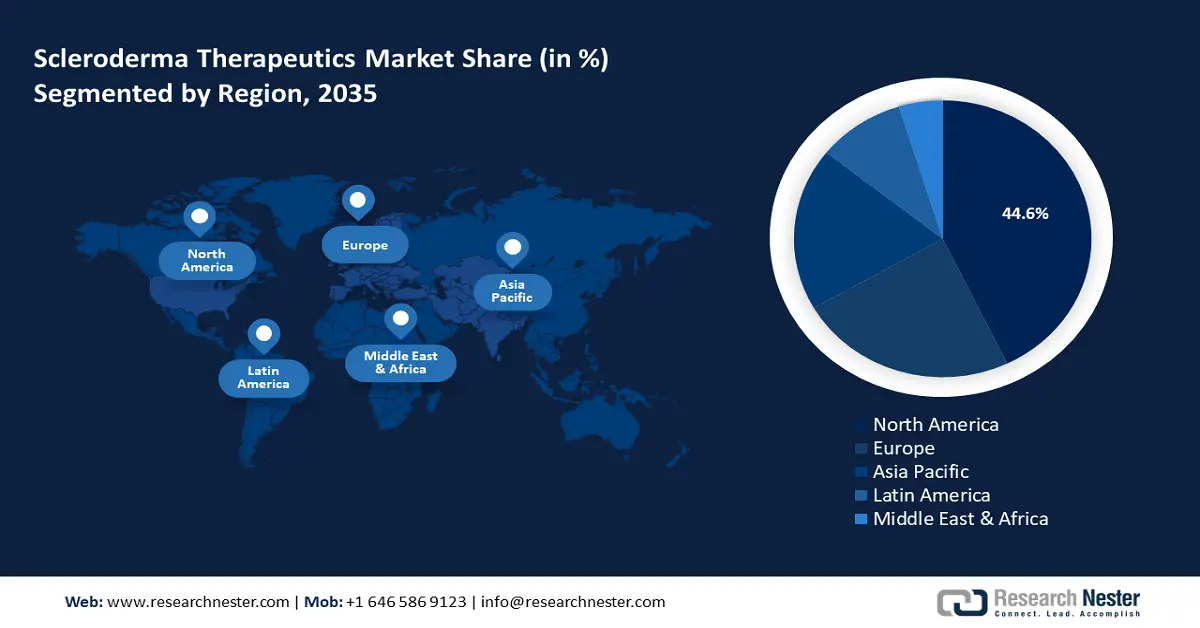
North America scleroderma therapeutics market is set to capture revenue share of over 44.6% by 2035. The market’s growth is attributed to the rising diagnosis of scleroderma in the region and an established supportive regulatory ecosystem. The market’s profit share is led by the U.S. and Canada in North America. Additionally, an advanced healthcare infrastructure in the region ensures patients are provided access to timely care leading to increased demands for scleroderma therapeutics. In January 2022, Paracine received U.S. FDA clearance to launch a trial in the U.S. in patients with hand dysfunction due to diffuse cutaneous scleroderma.
The U.S. registers the largest market share in the North America scleroderma therapeutics market. The country’s strong regulatory environment incentivizes innovation through programs such as the Orphan Drug Act. For instance, in September 2024, the calcium channel blocker Profervia of Aisa Pharmaceuticals was granted FDA orphan drug status as a treatment for scleroderma. The domestic market’s growth is assisted by the advancements in research led by major pharmaceutical companies in the region. An increase in scleroderma diagnosis benefits clinical trials with a high rate of patient enrolment benefiting advancements in new therapeutics.
The Canada market for the scleroderma therapeutics sector is poised to increase its revenue share by the end of 2035. The market benefits from rising awareness regarding autoimmune disease and the universal healthcare system in the country reducing patient’s economic burden. Non-profit organizations such as Scleroderma Canada are advocating for increased funding for therapeutics research and creating a network of patients diagnosed with the disease to form a community to offer urgent care. Additionally, the rise in cases of scleroderma in children is poised to increase demands for therapeutics. For instance, in July 2024, the Lancet Regional Health-Americas released a report stating scleroderma cases were on the rise in Quebec among children. The report additionally highlighted declining mortality rates but the uneven geographic distribution of cases requires tailored interventions.
Europe Market Analysis
The scleroderma therapeutics market in Europe is poised to register the fastest growth by the end of the forecast period. The growth of the market in Europe is attributed to collaboration between research institutions, private companies, and public health bodies leading to advancements in research. A favorable regulatory ecosystem in the region fostered by the European Medicines Agency (EMA) bolsters the market’s growth. France, Germany, and the United Kingdom are leading the revenue share in Europe. For instance, in August 2023, MediciNova was granted a patent in Europe for potential scleroderma treatment by the use of an investigational small molecule called MN-001 (Tipelukast) from the European Patent Office.
France is a leading market in the scleroderma therapeutics sector of Europe. The domestic market in the country benefits from government funding of research on rare diseases. The French National Plan for Rare Diseases from 2018 to 2023 focused on treatment for all and the next French National Plan for Rare Diseases is poised to be based on boosting R & D and innovation. The market is additionally boosted by the emphasis on early diagnostics and specialized care fueling demands for therapeutics. Patient support organizations such as Association des Sclérodermiques de France benefits the sector’s growth by raising awareness and pooling resources for better scleroderma care. In September 2024, French pharmaceutical powerhouse Sanofi stated their Tolebrutinib demonstrated 31% delay in time of onset of confirmed disability progression in non-relapsing secondary progressive scleroderma phase 3 study.
Germany is poised to increase its revenue share in the scleroderma therapeutics market of Europe by the end of the forecast period. The domestic market benefits from efforts to build a patient registry that is positioned to assist clinical trials. For instance, the German Network for Systemic Sclerosis (DNSS), i.e., interdisciplinary collaboration of 25 clinical centers focused on systemic sclerosis research, created a patient registry in 2023 and around 5000 patient cases have been recorded. Advanced care for scleroderma is provided in the country via specialized rheumatology centers improving access to therapeutics. The domestic market also benefits from research contributions from the Federation of European Scleroderma Associations (FESCA) and recent regulatory approvals of therapeutics for scleroderma care in Europe. For instance, in July 2023, the EMA granted FT011 of Certa orphan drug status in Europe with a 7-year market exclusivity if the therapy is approved.