Pharmaceutical Contract Manufacturing and Research Services Market Outlook:
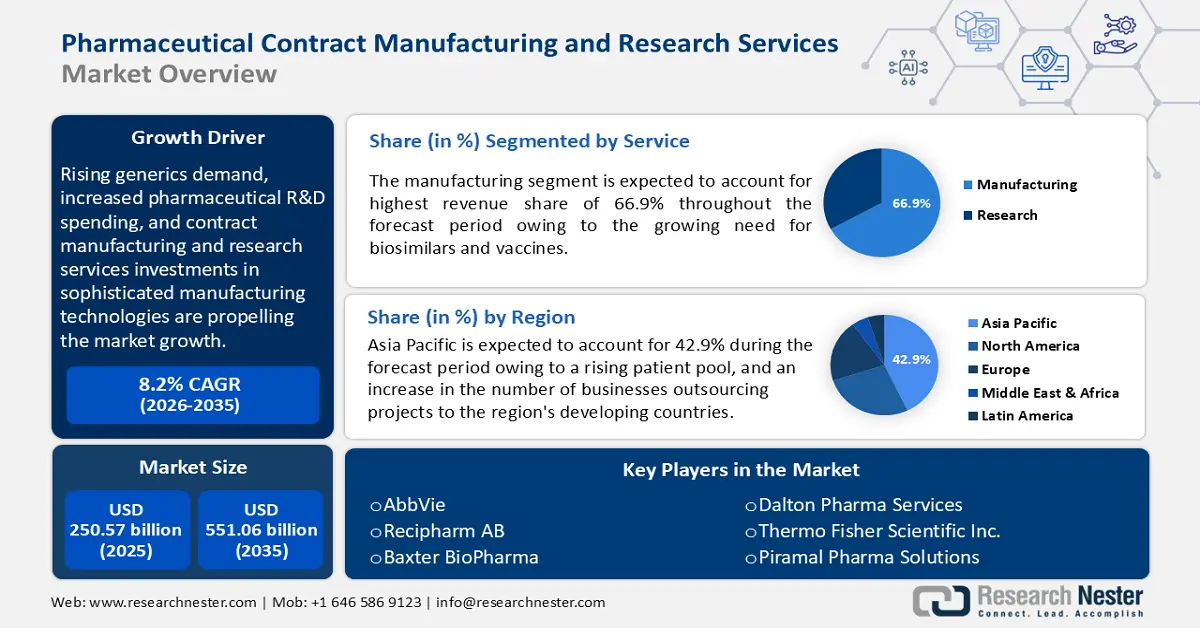
Pharmaceutical Contract Manufacturing and Research Services Market size was valued at USD 250.57 billion in 2025 and is set to exceed USD 551.06 billion by 2035, expanding at over 8.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of pharmaceutical contract manufacturing and research services is estimated at USD 269.06 billion.
Rising generics demand, increased pharmaceutical R&D spending, and contract manufacturing and research services investments in sophisticated manufacturing technologies are propelling the market forward. According to a report by Research Nester, in 2023, more than 5,500 pharmaceutical businesses had active R&D pipelines. Growing demand for biological therapies, a greater emphasis on specialty medicines, nuclear medicine sector expansion, and advances in cell and gene therapies are all expected to drive pharmaceutical contract manufacturing and research services market growth in the coming years.
Active Pharmaceutical Ingredient (API) CMOs are focused on navigating value chain challenges and ensuring best quality practices including Current Good Manufacturing Practice (CGMP) using corrective and preventative action (CAPA) by the FDA. In October 2023, Farmabios gained AIFA's current Good Manufacturing Practices (cGMP) approval to extend its production of Highly Potent APIs (HPAPIs), steroids, generics, and CDMO services.