Paroxysmal Nocturnal Hemoglobinuria Treatment Market Outlook:
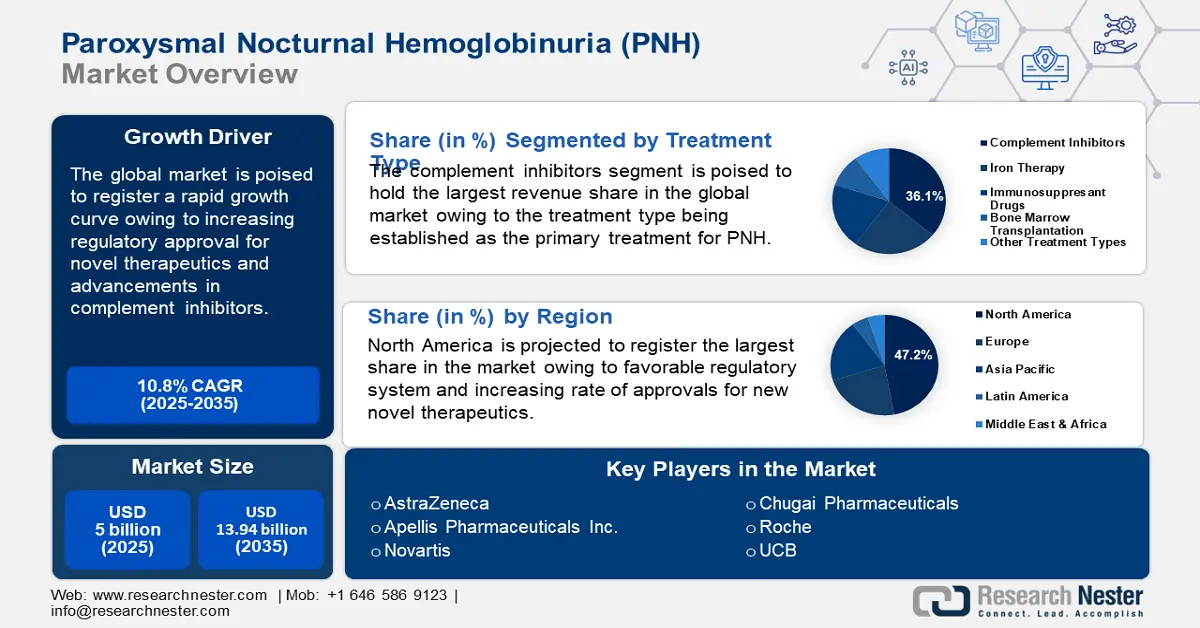
Paroxysmal Nocturnal Hemoglobinuria Treatment Market size was valued at USD 5 billion in 2025 and is expected to reach USD 13.94 billion by 2035, registering around 10.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of paroxysmal nocturnal hemoglobinuria treatment is assessed at USD 5.49 billion.
The market’s robust growth is attributed to the rising prevalence of rare blood disorders and advancements in therapeutic interventions, favoring companies in the healthcare sector to invest in developing, distributing, and selling advanced therapeutics for treatment. A paper published in the National Library of Medicine on inherited diseases of hemoglobin as an emerging global health burden predicted diseases such as paroxysmal nocturnal hemoglobinuria would cause a severe global health burden, especially in emerging economies.
PNH is a complement-mediated disease and requires targeted treatment. The global paroxysmal nocturnal hemoglobinuria (PNH) treatment market benefits from innovations in C5 inhibitors. For instance, in March 2020, a paper published in the National Library of Medicine stated that Crovalimab, a sequential monoclonal antibody recycling technology antibody was administered in small volumes once every 4 weeks and found complete terminal complement pathway inhibitions in patients with PNH, warranting the next phase of clinical development. An increasing rate of clinical approval for PNH treatment drugs such as Solaris (eculizumab), Ultomiris (ravulizumab), and Empaveli (pegcetacoplan) boosts the market’s growth. Market players are set to benefit from the increasing adoption of advanced diagnostic techniques assisting the early detection of PNH.
Rapid advancements in DNA sequencing assist early identification of PNH boosting the growth of the global paroxysmal nocturnal hemoglobinuria (PNH) treatment market as the demand for novel therapeutics rises. Additionally, industry stakeholders are focusing on strategic partnerships to improve distribution channels in emerging markets. A rising demand for PNH treatment has led to pharmaceutical companies pushing for regulatory approvals on novel therapies. New treatments receiving regulatory approvals boost the market’s growth as manufacturers, distributors, and end-users benefit from a positive treatment approval trend. For instance, in September 2024, Roche’s PIASKY received approval from the Food and Drug Administration (FDA) of the U.S. As global healthcare increases investment in gene therapy and precision medicine, new avenues are positioned to open in the paroxysmal nocturnal hemoglobinuria (PNH) treatment market driving the robust growth curve by the end of the forecast period.