Multiple Sclerosis Therapeutic Market Outlook:
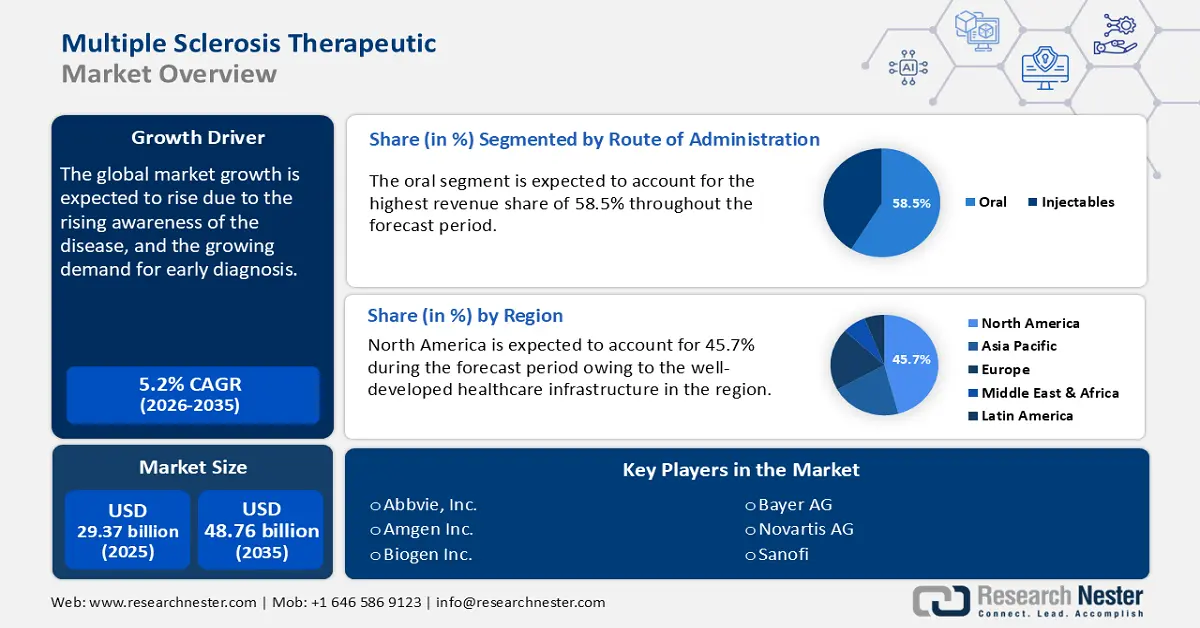
Multiple Sclerosis Therapeutic Market size was over USD 29.37 billion in 2025 and is projected to reach USD 48.76 billion by 2035, growing at around 5.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of multiple sclerosis therapeutic is evaluated at USD 30.74 billion.
The increasing prevalence of multiple sclerosis globally with its rising awareness is leading to earlier diagnosis and treatment. Advancements in medical research have further resulted in the development of innovative therapies, including biologics, monoclonal antibodies, and oral treatments. These treatments offer improved efficacy and patient convenience.
As per an article posted by WHO in August 2023, over 1.8 million people around the world suffer from multiple sclerosis (MS). The disease is primarily common among young adults and females. Several companies are engaging in R&D to accelerate the development and launch of next-generation treatments to address this rising concern. For instance, in December 2022, the U.S. FDA approved Briumvi to treat adults suffering from relapsing multiple sclerosis (RMS), including clinically isolated syndrome, relapsing-remitting MS, and active secondary-progressive MS. In February 2024, Neuraxpharm Group announced the launch of Briumvi in Europe. Additionally, the trend toward personalized medicines is gaining traction as patients and healthcare providers seek more tailored therapeutic approaches.