What is COVID-19?
A new form of the recently discovered coronavirus, COVID-19, has been spreading at a rapid rate globally. As of March 16, 2020, the World Health Organization confirmed a total of 167,511 confirmed cases of COVID-19, with 13,903 new cases and 6,606 deaths.
Coronavirus consists of a large family of viruses which infect both animals as well as humans. The transmission of COVID-19 is classified into local transmission or community spread and into imported cases.
The disease was first discovered in December 2019 in Wuhan, Hubei Province, China. It primarily affects the respiratory system, as a result of which, the virus is possibly spread from one person to another through respiratory droplets or close contact.
The Role of Pharmaceutical Companies in COVID-19 Vaccines and Drug Development
Since COVID-19 is a newly identified disease, there are no FDA-approved diagnostics, therapeutics or vaccines for it. However, as soon as the disease gained recognition globally, several leading pharmaceutical and biotechnology companies began the research and development in search of an effective coronavirus vaccine.
Sanofi and Regeneron Pharmaceuticals announced a new U.S.-based clinical program on March 16, 2020 for the evaluation of Kevzara® (sarilumab) in patients with severe COVID-19. Kevzara is a human monoclonal antibody that binds with the interleukin-6 (IL-6) receptor and inhibits the IL-6 pathway, which plays an important role in initiating the overactive inflammatory response in the lungs of COVID-19 infected patients. The trial will be conducted at various medical centers in New York. On March 9, 2020, AbbVie confirmed the company’s involvement in COVID-19 detection and treatment. The company intends to study the effects of HIV drug, Kaletra/Aluvia (lopinavir/ritonavir), for the treatment of the new coronavirus. AbbVie will be supporting clinical trials and research and working with the U.S. Food and Drug Administration, National Institute of Health, Centers for Disease Control and Prevention, Biomedical Advanced Research and Development Authority and European health authorities.
Gilead Sciences, Inc. revealed the initiation of its two Phase 3 clinical studies on February 26, 2020 to determine the efficacy and safety of investigational antiviral drug remdesivir in adults with COVID-19. The trials, beginning in March, would enroll around 1,000 patients majorly belonging to Asian countries and would evaluate two dosing durations of the drug, administered intravenously. Gilead’s investigational new drug, remdesivir, has received FDA review and acceptance for the treatment of COVID-19. On February 24, 2020, GlaxoSmithKline announced a research collaboration with Clover Biopharmaceuticals, a clinical-stage biotechnology company based in China. The collaboration aims to research and develop Clover’s protein based coronavirus vaccine candidate, COVID-19 S-Trimer. GSK will be providing its adjuvant technology to Clover’s promising vaccine candidate which would allow a higher production of vaccine doses.
What is COVID-19?
A new form of the recently discovered coronavirus, COVID-19, has been spreading at a rapid rate globally. As of March 16, 2020, the World Health Organization confirmed a total of 167,511 confirmed cases of COVID-19, with 13,903 new cases and 6,606 deaths.
Coronavirus consists of a large family of viruses which infect both animals as well as humans. The transmission of COVID-19 is classified into local transmission or community spread and into imported cases.
The disease was first discovered in December 2019 in Wuhan, Hubei Province, China. It primarily affects the respiratory system, as a result of which, the virus is possibly spread from one person to another through respiratory droplets or close contact.
The Role of Pharmaceutical Companies in COVID-19 Vaccines and Drug Development
Since COVID-19 is a newly identified disease, there are no FDA-approved diagnostics, therapeutics or vaccines for it. However, as soon as the disease gained recognition globally, several leading pharmaceutical and biotechnology companies began the research and development in search of an effective coronavirus vaccine.
Sanofi and Regeneron Pharmaceuticals announced a new U.S.-based clinical program on March 16, 2020 for the evaluation of Kevzara® (sarilumab) in patients with severe COVID-19. Kevzara is a human monoclonal antibody that binds with the interleukin-6 (IL-6) receptor and inhibits the IL-6 pathway, which plays an important role in initiating the overactive inflammatory response in the lungs of COVID-19 infected patients. The trial will be conducted at various medical centers in New York. On March 9, 2020, AbbVie confirmed the company’s involvement in COVID-19 detection and treatment. The company intends to study the effects of HIV drug, Kaletra/Aluvia (lopinavir/ritonavir), for the treatment of the new coronavirus. AbbVie will be supporting clinical trials and research and working with the U.S. Food and Drug Administration, National Institute of Health, Centers for Disease Control and Prevention, Biomedical Advanced Research and Development Authority and European health authorities.
Gilead Sciences, Inc. revealed the initiation of its two Phase 3 clinical studies on February 26, 2020 to determine the efficacy and safety of investigational antiviral drug remdesivir in adults with COVID-19. The trials, beginning in March, would enroll around 1,000 patients majorly belonging to Asian countries and would evaluate two dosing durations of the drug, administered intravenously. Gilead’s investigational new drug, remdesivir, has received FDA review and acceptance for the treatment of COVID-19. On February 24, 2020, GlaxoSmithKline announced a research collaboration with Clover Biopharmaceuticals, a clinical-stage biotechnology company based in China. The collaboration aims to research and develop Clover’s protein based coronavirus vaccine candidate, COVID-19 S-Trimer. GSK will be providing its adjuvant technology to Clover’s promising vaccine candidate which would allow a higher production of vaccine doses.
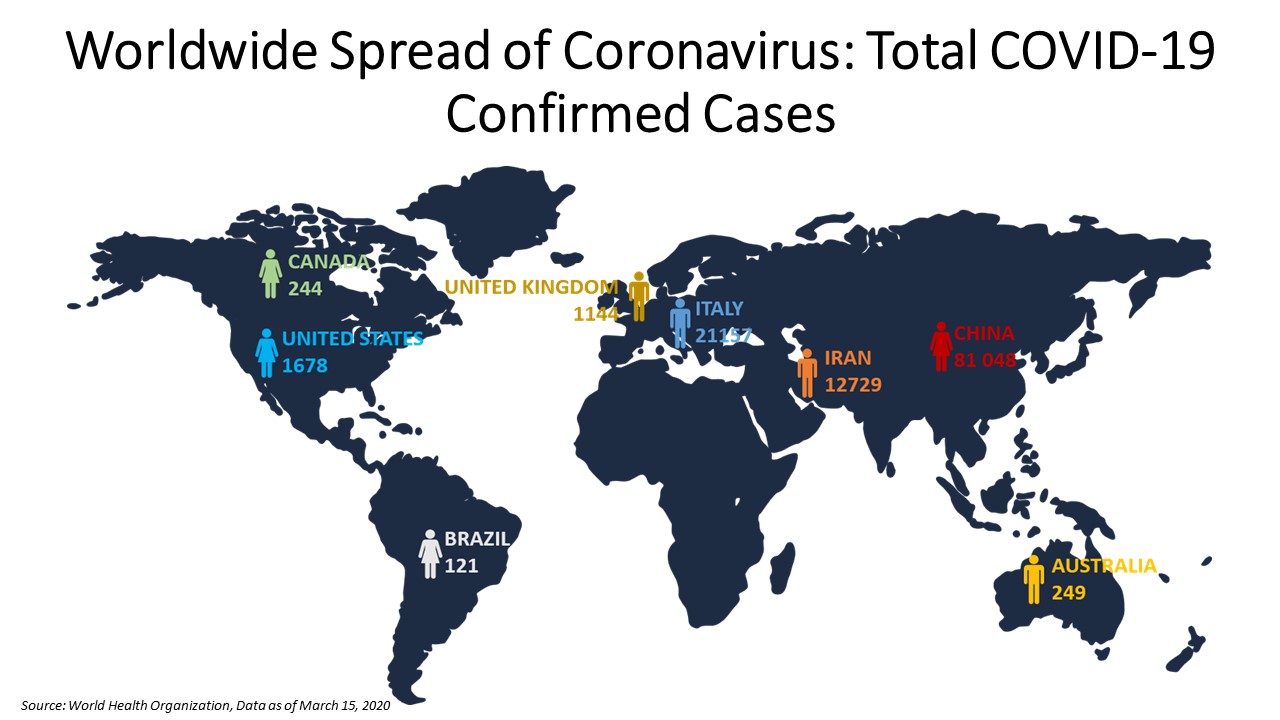
As of March 16, 2020, the Centers for Disease Control and Prevention reported 3,487 total cases in the United States, out of which, 205 cases were related to travel, 214 related to close contact and 3,068 cases were under investigation. In China, the highest number of cases were reported as per the W.H.O. with 81,077 confirmed cases as of March 16, 2020, out of which, 3,218 deaths took place.
According to the European Centre for Disease Prevention and Control, in Europe, the highest number of cases were reported in Italy with 27,980 total cases as of March 17, 2020. This was followed by 9,191 total cases in Spain, 6,633 total cases in France and 6,012 total cases in Germany.
The global COVID-19 outbreak was declared a pandemic by the World Health Organization on March 12, 2020.


