Wearable Cardioverter Defibrillators Market Outlook:
Wearable Cardioverter Defibrillators Market size was valued at USD 274.58 million in 2025 and is likely to cross USD 680.47 million by 2035, registering more than 9.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of wearable cardioverter defibrillators is assessed at USD 298.06 million.
The reason behind the growth is due to the rising incidence of cardiovascular diseases across the globe. Cardiovascular disease deaths have increased by more than 55% worldwide in the past 30 years.
Diabetes-related high blood sugar levels can harm the nerves that control the heart and blood vessels, resulting in a variety of cardiovascular diseases such as coronary artery disease, which affects the electrical conduction system in the heart, causing ventricular arrhythmias and atrial fibrillation. This increases the demand for common cardiac disease and rhythm monitoring, which is likely to boost market growth.
The growing technological advancement in cardiology are believed to fuel the market growth. For instance, artificial intelligence (AI) components are already being utilized in radiology and cardiology's backends including wearable cardiac monitoring devices and point-of-care (POC) triage apps that are being utilized with wearables or smartphone-based apps that record ECG to automatically detect arrhythmias and notify patients.
Key Wearable Cardioverter Defibrillators Market Insights Summary:
Regional Highlights:
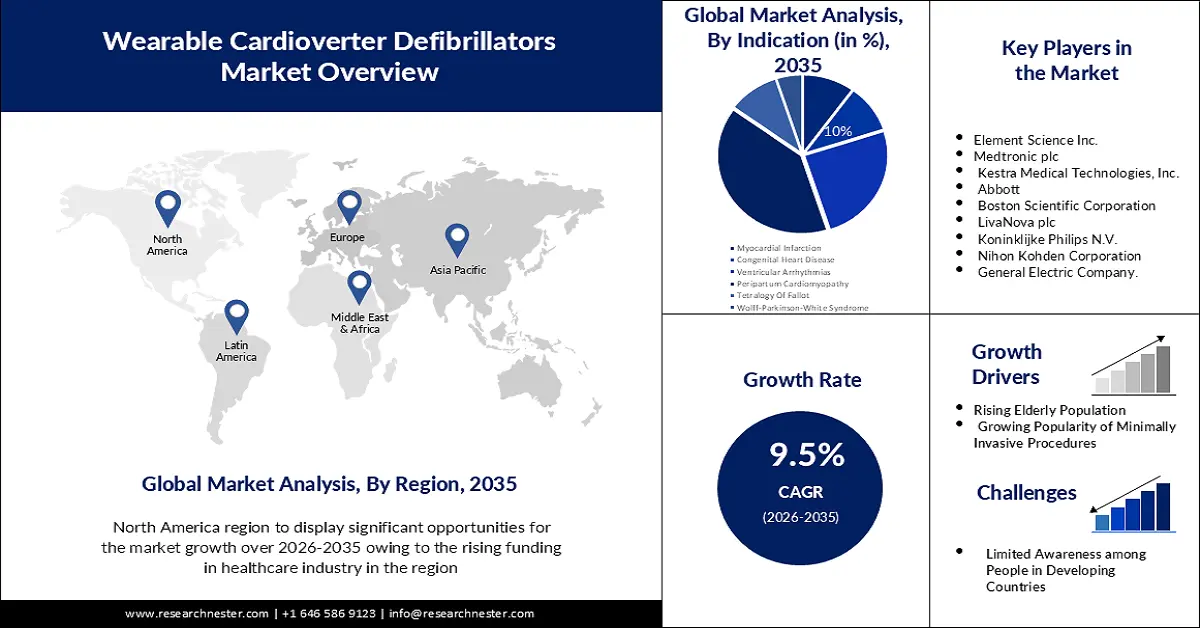
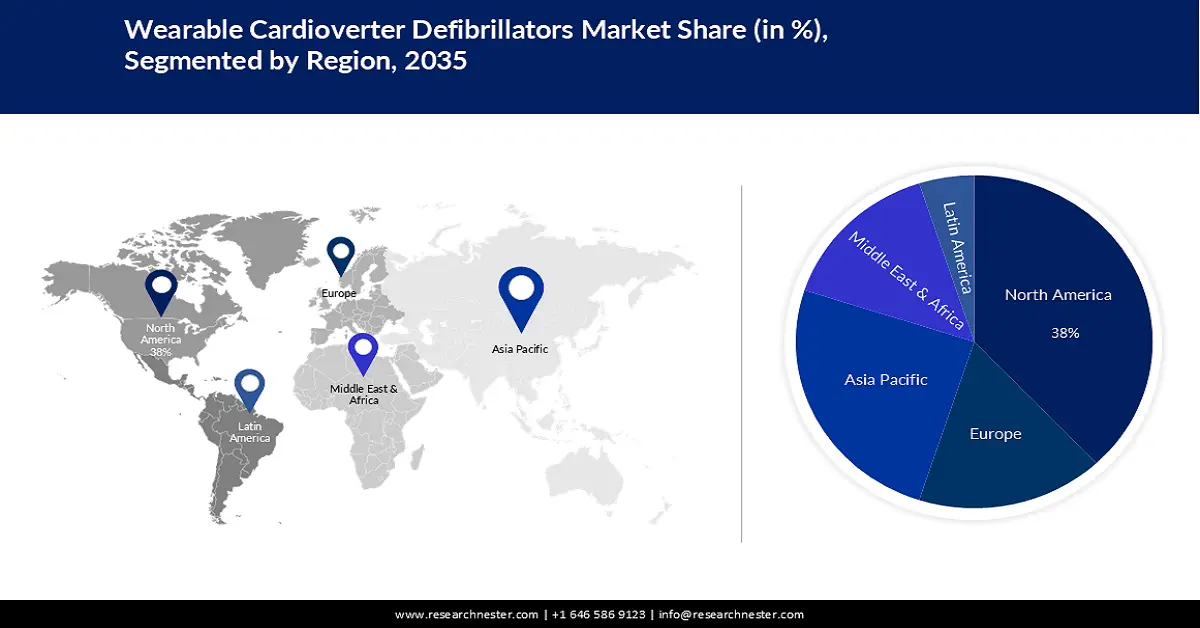
- By 2035, North America is anticipated to command a 38% share of the wearable cardioverter defibrillators market, supported by expanded R&D capabilities and increasing healthcare expenditure owing to advanced device innovation.
- By 2035, the APAC region is projected to secure the second-largest share, underpinned by rising medical-device investments and supportive government programs encouraging high-quality healthcare access across the region.
Segment Insights:
- By 2035, the peripartum cardiomyopathy segment is projected to account for around 40% share of the wearable cardioverter defibrillators market, propelled by its growing need for effective arrhythmia management in high-risk patients.
- Over 2026-2035, the home care settings segment is expected to gain a notable share, sustained by rising preference for at-home treatment supported by virtual medical assistance.
Key Growth Trends:
- Rising Elderly Population
- Growing Popularity of Minimally Invasive Procedures
Major Challenges:
- Limited Awareness among People in Developing Countries
- Stringent Government Regulations can Pose Challenge for Manufacturers
Key Players: ZOLL Medical Corporation, Element Science Inc., Medtronic plc, Kestra Medical Technologies, Inc., Abbott, Boston Scientific Corporation, LivaNova plc, Koninklijke Philips N.V., Nihon Kohden Corporation and General Electric Company.
Global Wearable Cardioverter Defibrillators Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 274.58 million
- 2026 Market Size: USD 298.06 million
- Projected Market Size: USD 680.47 million by 2035
- Growth Forecasts: 9.5%
Key Regional Dynamics:
- Largest Region: North America (38% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, United Kingdom, China
- Emerging Countries: India, South Korea, Brazil, Singapore, Mexico
Last updated on : 19 November, 2025
Wearable Cardioverter Defibrillators Market - Growth Drivers and Challenges
Growth Drivers
- Rising Elderly Population – The number of cardiac arrests among the elderly is expected to rise in the coming decades, as men and the elderly are most vulnerable to cardiac arrest. According to the World Health Organization (WHO), the number of people aged 60 and up will double to 2.1 billion worldwide by 2050.
- Growing Popularity of Minimally Invasive Procedures- The wearable cardioverter-defibrillator (WCD) is a temporary, non-invasive device that is often given to individuals who are at high risk of arrhythmia.
- Spiking Incidence of Diabetes- Cardiovascular disease is two to four times more common in people with diabetes than in the general population as high blood sugar levels have the potential to harm heart-controlling neurons and blood arteries over time. By 2030, it is expected that the prevalence of type 2 diabetes will rise to over 7070 cases per 100,000 people worldwide.
- Increasing Launch of New Products by Major Market Players- For instance, in August 2021, Kestra Medical Technologies received approval from the FDA for an Assure wearable cardioverter defibrillator (WCD) system with integrated sensors, a cardiac rhythm monitor, and a miniaturized automated external defibrillator, to assess a patient's cardiac rhythm, and safely provide defibrillation therapy to return a patient's heart to normal to safeguard patients at risk of sudden cardiac death (SCD).
- Growing Consumption of Alcohol- One of the main causes of cardiac arrest is alcohol consumption, which also weakens the heart and can cause hypertension and cardiomyopathy, a condition that damages the heart muscle. For instance, every year, alcohol use results in over 2 million premature deaths worldwide.
Challenges
- Limited Awareness among People in Developing Countries - In developing countries, the knowledge about the cardioverter-defibrillator is still minimal, which may act as a barrier to the development of the wearable cardioverter defibrillators market. Furthermore, in various low-income countries, there are no strict standards and regulations to evaluate the wearable cardioverter defibrillators' safety and quality. The poorly developed standards across multiple nations have further hindered the usage of wearable cardioverter defibrillators.
- Stringent Government Regulations can Pose Challenge for Manufacturers
- High Cost of Wearable Cardioverter Defibrillators can Limit the Adoption and Access for Certain Individuals
Wearable Cardioverter Defibrillators Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
9.5% |
|
Base Year Market Size (2025) |
USD 274.58 million |
|
Forecast Year Market Size (2035) |
USD 680.47 million |
|
Regional Scope |
|
Wearable Cardioverter Defibrillators Market Segmentation:
Indication Segment Analysis
The peripartum cardiomyopathy segment is estimated to hold 40% share of the global wearable cardioverter defibrillators market in the coming years. Pregnancy-associated cardiomyopathy, commonly known as peripartum cardiomyopathy, is a kind of systolic heart failure (HF) with a reduced left ventricular ejection fraction (LVEF) that affects women who are pregnant or in the months following delivery. Moreover, it is an unusual kind of heart failure that usually manifests with decreased ejection fraction (HFrEF) and is a potentially fatal disorder. This is expected to fuel the demand for a wearable cardioverter defibrillator as it can safely record and stop life-threatening arrhythmias, making it useful for illnesses such as peripartum cardiomyopathy.
End-user Segment Analysis
The home care settings segment is set to garner a notable share. It is the preferred way of treating patients to preserve their independence and remain in the comfort of their surroundings. Moreover, more people nowadays are focusing more on spending on resources for proper care at home. Furthermore, with the advancement in medical technology, physicians can now assist their patients virtually, which has further led to the increasing acceptance of home care settings. The hospital segment has accounted for a significant market share in the past years and is anticipated to play a key role in the growth of the market on account of an increasing number of hospitals providing numerous options for treatment across developing countries.
Demography Segment Analysis
The adults segment in the wearable cardioverter defibrillators market is anticipated to gain a robust revenue share of 79% by 2035. People with high risk of cardiovascular diseases usually include adults with hypertension, diabetes, obesity, and other diseases which may increase the demand for wearable cardioverter defibrillators as they can be used to monitor cardiovascular conditions.
Our in-depth analysis of the global market includes the following segments:
|
Indication |
|
|
Demography |
|
|
End-User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Wearable Cardioverter Defibrillators Market - Regional Analysis
North American Market Insights
North America industry is expected to hold largest revenue share of 38% by 2035. As a result, more resources are available in the region for research and development of more advanced, and reliable wearable cardioverter defibrillators which would also make these devices cost-effective in the region. In 2021, U.S. health spending climbed by more than 2% to reach over USD 4 trillion.
APAC Market Insights
The APAC wearable cardioverter defibrillators market is estimated to be the second largest, during the forecast timeframe led by increase in investment towards the medical device sector from government and private entities to maintain the highest quality of services. The Indian government supports the production of medical devices in the country by offering various programs and subsidies under several ministries which has spread the word in the community about the value of leading a healthy lifestyle and the right of everyone to get high-quality medical care. This has led to an increase in demand for various advanced medical devices such as wearable cardioverter defibrillators in the region.
Wearable Cardioverter Defibrillators Market Players:
- ZOLL Medical Corporation
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Element Science Inc.
- Medtronic plc
- Kestra Medical Technologies, Inc.
- Abbott
- Boston Scientific Corporation
- LivaNova plc
- Koninklijke Philips N.V.
- Nihon Kohden Corporation
- General Electric Company.
Recent Developments
- Medtronic plc announced that it has obtained approval from the U.S. Food and Drug Administration (FDA) for Aurora EV-ICD MRI SureScan (Extravascular Implantable Cardioverter-Defibrillator) and Epsila EV MRI SureScan to offer the advantages of conventional, transvenous ICDs in terms of saving lives by providing anti-tachycardia pacing (ATP), backup (pause-prevention) pacing therapy, and life-saving defibrillation.
- Abbott received approval from the U.S. Food and Drug Administration (FDA) for Gallant implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) devices with Bluetooth technology and a new patient smartphone app for better remote monitoring for patients with cardiac rhythm abnormalities.
- Report ID: 874
- Published Date: Nov 19, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Wearable Cardioverter Defibrillators Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




