Trisenox Market Outlook:
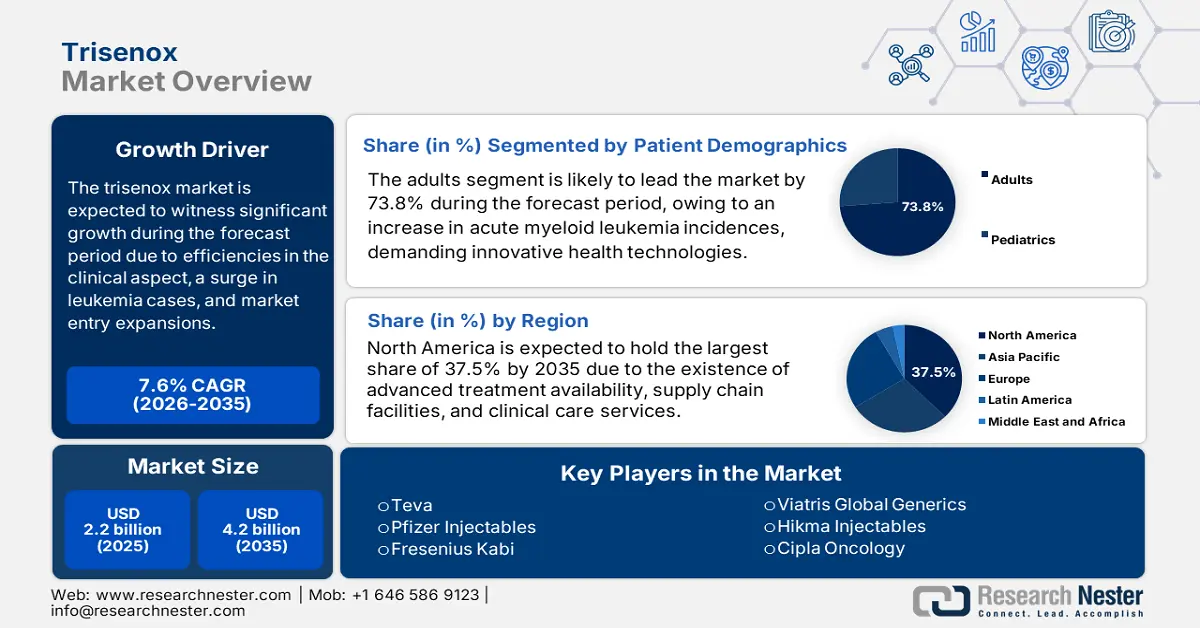
Trisenox Market size was USD 2.2 billion in 2025 and is anticipated to reach USD 4.2 billion by the end of 2035, increasing at a CAGR of 7.6% during the forecast period, i.e., 2026-2035. In 2026, the industry size of trisenox is evaluated at USD 2.3 billion.
The trisenox market’s development is not only fueled by a single factor, but by a confluence of demographic, clinical, and economic trends. These include irreplaceable and established clinical efficacy, an increase in leukemia cases, along with its diagnosis, expansion into emerging nations, and continuous clinical research for extending labels. According to an article published by the Leukemia and Lymphoma Society in 2024, an estimated one person in every 3 minutes is diagnosed with leukemia in the U.S., with a combined 187,740 people. Additionally, the latest cases of leukemia, myeloma, and lymphoma account for 9.4%, accounting for 2,001,140 new cancer cases, all of which were diagnosed in the country as of 2024, thus suitable for the market’s demand..
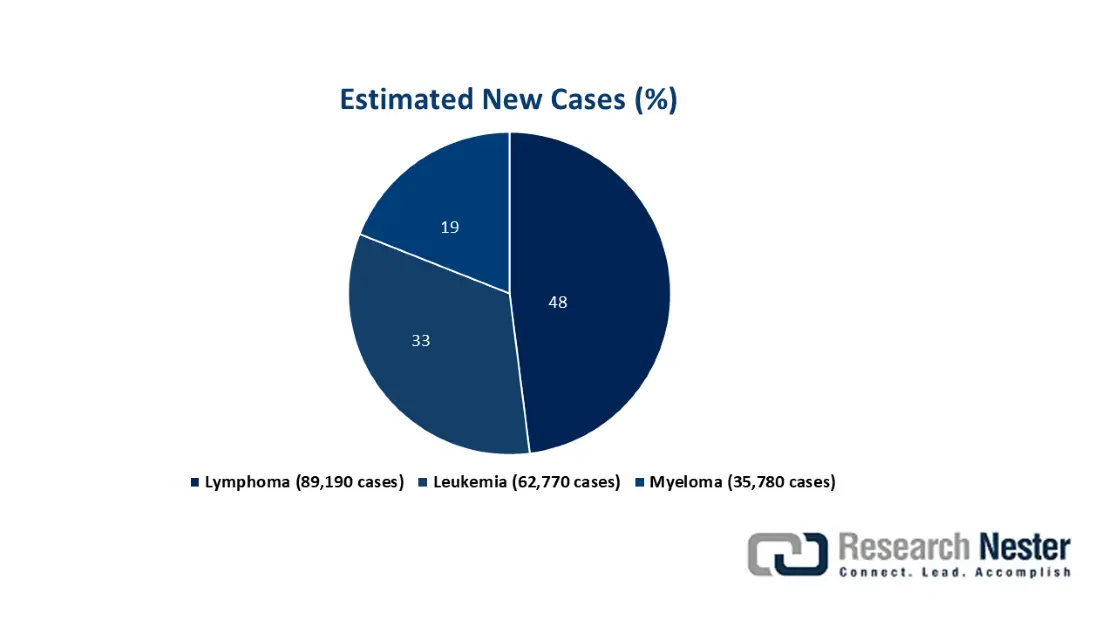
Source: Leukemia and Lymphoma Society, 2024
Moreover, the aspect of robust integration into treatment guidelines, as well as genericization and patients' expiry, are also driving the market’s exposure globally. Complete blood count, peripheral blood smear, flow cytometry, bone marrow biopsy, molecular testing, immunophenotyping, and RT-PCR test are a few treatments that are suitable for aiding leukemia. As per the May 2025 National Cancer Institute report, for newly diagnosed acute myeloid leukemia (AML), almost 20% of cells are blasts in the bone marrow, thereby denoting leukemia symptoms. Besides, chemotherapy and radiation therapy are the most hyped solutions for treatment, which is positively impacting the market’s exposure.
Key Trisenox Market Insights Summary:
Regional Highlights:
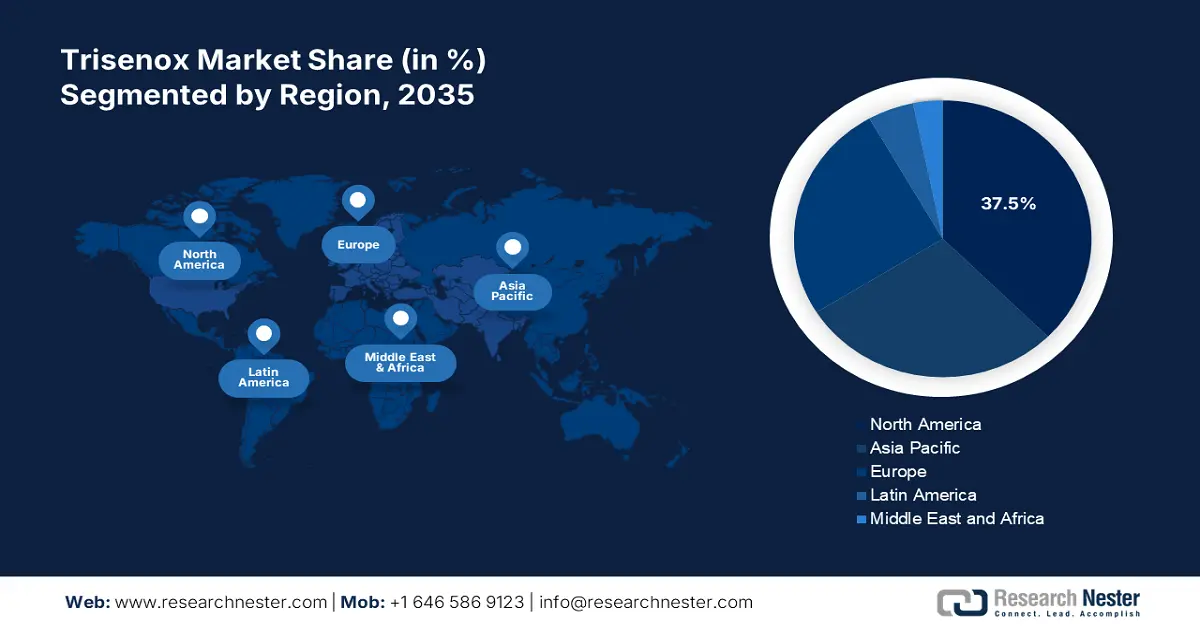
- North America in the trisenox market is projected to dominate with a 37.5% share by 2035, attributed to robust regulatory and clinical care frameworks, enhanced treatment availability, and efficient supply chain operations.
- Europe is expected to capture a 25.7% share by 2035, owing to favorable regulatory authorizations, market accessibility, and cross-border procurement policies.
Segment Insights:
- The adults segment in the trisenox market is projected to hold the largest share of 73.8% by 2035, propelled by the growing prevalence of leukemia cases among adult populations across various countries.
- The acute promyelocytic leukemia segment is anticipated to secure a 68.4% share by 2035, driven by the rising deterioration in clinical status and increasing adoption of advanced technologies for patient management.
Key Growth Trends:
- Novel formulation development
- Diagnostic awareness campaigns
Major Challenges:
- Fragmented and complicated regulatory pathways
- Real-world evidence demand by payers
Key Players: Teva Pharmaceutical Industries Ltd. (Israel), Pfizer Inc. (Hospira) (U.S.), Fresenius Kabi AG (Germany), Mylan N.V. (now part of Viatris Inc.) (U.S.), Hikma Pharmaceuticals PLC (UK/Jordan), Cipla Ltd. (India), Dr. Reddy's Laboratories Ltd. (India), Sun Pharmaceutical Industries Ltd. (India), Novartis AG (Sandoz) (Switzerland), Aurobindo Pharma Ltd. (India), Intas Pharmaceuticals Ltd. (India), Jiangsu Hengrui Medicine Co., Ltd. (China), Gland Pharma Ltd. (India), Apotex Inc. (Canada), Kelun Pharmaceutical Co., Ltd. (China).
Global Trisenox Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 2.2 billion
- 2026 Market Size: USD 2.3 billion
- Projected Market Size: USD 4.2 billion by 2035
- Growth Forecasts: 7.6% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (37.5% Share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, Germany, United Kingdom, France, Japan
- Emerging Countries: China, India, South Korea, Brazil, Australia
Last updated on : 5 September, 2025
Trisenox Market - Growth Drivers and Challenges
Growth Drivers
- Novel formulation development: This readily focuses on novel drug delivery technology for increasing the efficacy, along with diminishing side effects related to herbal medicines, which is effectively driving the trisenox market globally. According to an article published by the National Cancer Institute in March 2025, there are almost 6,100 AML cases, as well as 1,400 deaths caused by this condition, particularly in the U.S. Therefore, to overcome this, novel chemotherapeutic agents and dasatinib adoption, which is a tyrosine kinase inhibitor, can be utilized, thus catering to the market’s development.
- Diagnostic awareness campaigns: These campaigns are essential in the healthcare sector, owing to the aspect of early disease detection, promote preventive measures, and effectively facilitate timely medical intervention. According to the June 2025 NIH report, 32% of adults in America are readily willing to be a part of cancer-based clinical trials, followed by 28% considering it based on reservation facilities. Therefore, by conducting awareness campaigns in the form of clinical studies or trials, awareness is created, which can modify patients’ attitudes towards enrollment and participation.
- Strategic expansion into emerging nations: This growth driver is crucial for the trisenox market, owing to the ability to expand accessibility, improve quality, and reduce health expenses. For instance, in July 2025, KKR & Co. Inc. successfully acquired a majority of ownership-based stake in HealthCare Royalty Partners (HCRx). This tactical partnership enabled KKR to augment its abilities in credit investing and biopharma royalty, along with extending the organization’s footprint in the life sciences ecosystem across different nations.
Historical 2019-2023 Leukemia Cases, Deaths, and Survival
|
Year |
Rate of New Cases- SEER 8 |
Rate of New Cases- SEER 12 |
Death Rate- U.S. |
5-Year Relative Survival- SEER 8 |
||||
|
Observed |
Modeled Trend |
Observed |
Modeled Trend |
Observed |
Modeled Trend |
Observed |
Modeled Trend (%) |
|
|
2019 |
14.6 |
14.6 |
14.1 |
13.9 |
6.0 |
6.0 |
- |
70.2 |
|
2020 |
13.6 |
14.5 |
13.2 |
13.8 |
6.0 |
5.9 |
- |
71.2 |
|
2021 |
14.7 |
14.4 |
13.8 |
5.8 |
5.8 |
19.19 |
- |
71.6 |
|
2022 |
14.3 |
14.3 |
13.5 |
13.7 |
5.7 |
5.7 |
- |
72.1 |
|
2023 |
- |
- |
- |
- |
5.6 |
5.6 |
- |
- |
Source: National Cancer Institute, 2025
Chemotherapy and Oral Tyrosine Kinase Inhibitor (TKI) Initiation for Curing Leukemia
|
Milestones |
Implications |
|
3 months |
BCR-ABL1 [International Scale (IS)] at ≤10 percent and/or ≤35% Ph-positive metaphase cells |
|
6 months |
BCR-ABL1 (IS) at ≤1 percent or/and 0 % Ph-positive metaphase cells |
|
1 year |
BCR-ABL1 (IS) ≤0.1 percent |
Challenges
- Fragmented and complicated regulatory pathways: Centralized bodies, such as PDMA and EMA, readily provide marketing authorization, whereas true market accessibility is effectively administered by national and sub-national reimbursement policies. Besides, every country has its respective health technology, price strategy, and formulary committee, and owing to this, all these entities demand separate negotiations, meetings, and submissions. Therefore, these are fragmentations, wherein manufacturers witness delay by months regarding a drug’s efficacy and safety, thereby negatively impacting the trisenox market globally.
- Real-world evidence demand by payers: This aspect has moved beyond an initiative to emerge as one of the standard requirements for market accessibility, along with reimbursement retention. Besides, payers are readily skeptical about clinical trials, and need proof to ensure value and effectiveness in heterogeneous populations within healthcare systems. This frequently necessitates the development of expensive patient registries, extended data collection protocols, and continuous pharmacovigilance studies, particularly for satisfying payer questions. Besides, this is a post-launch burden for manufacturers and producers, demanding long-lasting investments, which has created a gap in the trisenox market.
Trisenox Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
7.6% |
|
Base Year Market Size (2025) |
USD 2.2 billion |
|
Forecast Year Market Size (2035) |
USD 4.2 billion |
|
Regional Scope |
|
Trisenox Market Segmentation:
Patient Demographics Segment Analysis
The adults segment in the trisenox market is anticipated to account for the largest share of 73.8% by the end of 2035. The segment’s development is highly propelled by a surge in leukemia incidence among this category of the population across different nations. As per an article published by the American Cancer Society in March 2025, an estimated 1 out of 3 leukemia in adults constitutes acute myeloid leukemia, which accounts for almost 1% of overall cancer cases. This condition is extremely common among men in comparison to women, but the average lifetime risk of getting affected with AML is ½ of 1%, thus enhancing the market demand for adults.
Application Segment Analysis
The acute promyelocytic leukemia segment in the trisenox market is expected to garner the second-largest share of 68.4% during the forecast timeline. The segment’s growth is highly driven by increased deterioration in clinical status, along with the demand for innovative technologies to evaluate and manage patients with this condition. According to an article published by the NLM in January 2025, the RAR-alpha gene readily promotes different genetic expressions, and in almost 90% to 95% of cases, this condition results from a t (15;17) (q22;q21) translocation, which leads to tail fusion of the promyelocytic leukemia (PML) gene, thus enhancing the market’s demand globally.
Distribution Channel Segment Analysis
The hospitals segment is expected to hold the third-largest share of 61.7% in the trisenox market by the end of the projected duration. The segment’s growth is highly fueled by its direct connection with the clinical administration protocol. Besides, arsenic trioxide is an effectively utilized medication to aid APL, and this administration is readily associated with critical side effects, which include cardiac QT prolongation and differentiating syndromes. Meanwhile, ongoing management, treatment initiation, and diagnosis frequently take place within hospitals, due to which the segment’s exposure is increasing internationally.
Our in-depth analysis of the trisenox market includes the following segments:
|
Segment |
Subsegments |
|
Patient Demographics |
|
|
Application |
|
|
Distribution Channel |
|
|
Delivery Method |
|
|
Product Type |
|
|
End user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Trisenox Market - Regional Analysis
North America Market Insights
North America in the trisenox market is expected to be the most dominating region, garnering the highest share of 37.5% by the end of 2035. The market’s growth in the region is highly attributed to regulatory and clinical care services, treatment availability, supply chain distributions, and competitive landscape. As per the September 2023 White House government report, the U.S. was short of 15 cancer drugs, owing to issues in manufacturing and supply chains. However, to combat this, cooperation among manufacturers and healthcare systems with the FDA resulting in ensuring an estimated 400,000 patients with standard treatments every month, thus suitable for the overall market.
The trisenox market in the U.S. is growing significantly, owing to reimbursement policies, pricing strategies, outpatient administration, and legal reforms. Section 340B of the Public Health Service Act requires the participation of pharmaceutical manufacturers to distribute outpatient drugs by collaborating with Medicaid. In this regard, the January 2025 American Hospital Association report stated that, as of 2022, this particular drug program provided discounts, which accounted for 3% of drug organizations’ international revenues. In addition, the program has readily driven companies’ decisions regarding drug pricing, which is positively impacting the market in the country.
The trisenox market in Canada is also growing due to reimbursement formularies, administrative drug reviews, procurement negotiations, accessibility policies, and government funding for cancer cases. As per an article published by NLM in April 2022, cancer-based expense in the country was CAD 26.2 billion within the past 4 years, of which 30% were borne by patients. In addition, there was an increase in the country’s economic burden, during which patients and their families’ expenses amount to nearly CAD 4.8 billion in the same duration, thereby denoting a huge growth opportunity for the market.
Cancer Incidence Among Males and Females in North America
|
Country Name |
Both Gender |
Age-Standardized Rates |
|
U.S. |
2,380,189 |
367.0 |
|
Canada |
292,098 |
345.9 |
|
Mexico |
207,154 |
140.9 |
|
Cuba |
49,688 |
220.8 |
|
Guatemala |
17,801 |
121.8 |
|
Costa Rica |
13,325 |
177.7 |
|
Jamaica |
7,500 |
199.6 |
|
Bahamas |
955 |
192.7 |
Source: World Cancer Research Fund 2025
Europe Market Insights
Europe in the trisenox market is projected to be the fastest-growing region, accounting for a share of 25.7% during the forecast period. The market’s growth in the region is propelled by regulatory authorization, market accessibility, negotiations in procurement, and cross-border policies. As stated in the March 2024 European Journal of Political Economy report, the region tends to spend 14% of gross domestic product (GDP), accounting for €1.9 trillion yearly, for public procurement, which is readily allocated by over 250,000 public authorities. Besides, contract price, probability, and rebate of small and medium enterprises in the region have revealed lower discretion practices in connection with high contract prices, which positively impacts the market’s distribution.
The trisenox market in Germany is steadily growing, owing to free pricing and automatic accessibility, early benefit assessment, the statutory health insurance (SHI) funding system, and off-label clinical administration. According to the April 2025 Federal Ministry of Health data report, the SHI provides suitable health protection to nearly 90% of the population, and over 70 million citizens are successfully insured with the SHI fund to achieve suitable medical care facilities. Besides, the SHI operation depends on solidarity, denoting that 100 health insurance funds effectively grant necessary medical services to paying members, as well as an estimated 16 million people in the country.
The trisenox market in France is also growing due to pricing and reimbursement policies, rapid hospital funding, the existence of the National Cancer Institute (INCa) strategy, and mandatory revenue capping. In this regard, as stated in the Cancer Grand Challenges organization report, the French National Cancer Institute in September 2023, declared its €10.0 million partnership with Cancer Research UK to assist in funding global researchers. The objective was to overcome tough challenges in the cancer field through the Global Cancer Challenges strategy. Therefore, with this deal, there has been a huge growth opportunity for the market in the country.
Contract Price, Rebate, and Probability of an SME in Europe
|
Components |
All Countries |
Low GR |
High GE |
|
Contract Price: Law Practice |
-6.1 (4.5) |
-0.4 (8.9) |
-8.6 (5.3) |
|
6.6 (1.8) |
6.2 (2.0) |
9.3 (3.9) |
|
|
Rebate: Law Score Practice Score |
6.2 (2.3) |
8.7 (7.4) |
5.9 (2.4) |
|
-8.7 (1.1) |
-10.0 (1.0) |
-7.1 (2.0) |
|
|
Probability of an SME winning a contract: Law Practice |
0 (0.3) |
0.2 (1.5) |
-0.2 (0.2) |
|
1.8 (0.6) |
2.7 (0.6) |
0.6 (0.3) |
Source: European Journal of Political Economy, March 2024
APAC Market Insights
Asia Pacific in the trisenox market is expected to hold a considerable share of 28.6% by the end of the projected timeline. The market’s upliftment in the region is highly driven by a surge in the aging population, improved diagnostic capabilities, heterogeneous purchasing power, and localized generic manufacturing and approvals. As per a data report published by APAC Med in April 2022, generous investments in medical diagnostic technologies through the Universal Health Coverage (UHC) have contributed USD 2.5 trillion to the region. Besides, countries in the region, such as Odisha, which suffer almost 25% of India’s natural disasters, have evolved into a decentralized healthcare model, thus suitable for the overall market.
The trisenox market in China is gaining increased traction, owing to the presence of the National Reimbursement Drug List (NRDL), volume-based procurement programs, tiered hospital systems, and the Made in China policy. In this regard, the June 2023 NLM article stated that there has been an upsurge in the country’s healthcare spending, amounting to RMB 7.2 trillion over the past 5 years, which represented 7.1% of the GDP. This has readily driven social health insurance schemes, while out-of-pocket expenses constituted an estimated 30% of overall healthcare spending, thus suitable for bolstering the market in the country.
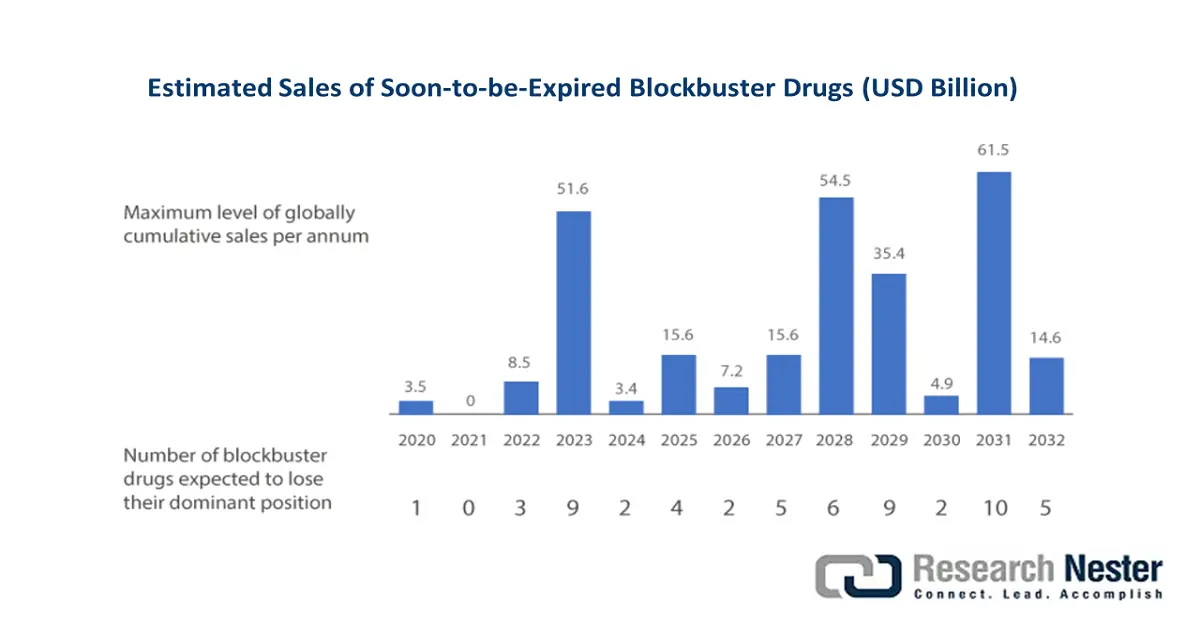
The trisenox market in South Korea is gaining equal importance due to positive listing by HIRA-NHIS, external reference pricing, competitive biosimilar, generic environment, and an increase in the standard care guideline adoption. According to the June 2023 Invest Korea Organization report, the biotechnology industry in the country amounted to KRW 21 trillion, denoting a 17.8% increase over the past 5 years. In addition, there has been a surge in production rates, along with export value by 11.7% and 14.8%. Besides, biotech organizations and regional pharmaceuticals increased their R&D activities to develop biologic products, resulting in increased sales of soon-to-be-expired blockbuster drugs.
Key Trisenox Market Players:
- Teva Pharmaceutical Industries Ltd. (Israel)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Pfizer Inc. (Hospira) (U.S.)
- Fresenius Kabi AG (Germany)
- Mylan N.V. (now part of Viatris Inc.) (U.S.)
- Hikma Pharmaceuticals PLC (UK/Jordan)
- Cipla Ltd. (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Novartis AG (Sandoz) (Switzerland)
- Aurobindo Pharma Ltd. (India)
- Intas Pharmaceuticals Ltd. (India)
- Jiangsu Hengrui Medicine Co., Ltd. (China)
- Gland Pharma Ltd. (India)
- Apotex Inc. (Canada)
- Kelun Pharmaceutical Co., Ltd. (China)
The international trisenox market is severely competitive, consolidated, and effectively dominated by established agile generic manufacturers, along with pharmaceutical giants. Notable key players, including Pfizer and Teva, have effectively leveraged extended international distribution networks, as well as broad product portfolios. Besides, tactical strategies are readily focused on achieving administrative clearance for generics in emerging nations, enhancing supply chains to diminish production expenses, and successfully engaging in value-specific contracting with players in developed countries, thereby suitable for bolstering the trisenox market internationally.
Here is a list of key players operating in the global market:
Recent Developments
- In March 2024, Bristol Myers Squibb notified that the U.S. FDA successfully cleared Breyanzi, which is a CD19-directed chimeric antigen receptor (CAR) T cell therapy, for treating adult patients affected with refractory chronic lymphocytic leukemia (CLL).
- In December 2024, Accenture collaborated with HealthCare Global Enterprises Limited (HCG) to escalate cancer research and care solutions by utilizing advanced AI, such as deep learning and generative AI.
- Report ID: 8061
- Published Date: Sep 05, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Trisenox Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




