Tricuspid Insufficiency Treatment Market Outlook:
Tricuspid Insufficiency Treatment Market size was over USD 778.33 million in 2025 and is projected to reach USD 1.57 billion by 2035, growing at around 7.3% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of tricuspid insufficiency treatment is assessed at USD 829.47 million.
The world's growing need for solutions to tricuspid stenosis and regurgitation of the tricuspid valve is responsible for the market's expansion. In the United States, 1.6 million people, and 3.0 million people in Europe have tricuspid regurgitation (TR).
In addition to these, a significant element anticipated to propel market expansion in the ensuing years is the high preference for tricuspid insufficiency treatment in clinical studies conducted by heart surgeons. Moreover, well-known producers in developed countries are placing a strong emphasis on acquisitions as a means of growing their regional footprint and diversifying their product lines, both of which are expected to present profitable prospects for the industry in the near future.
Key Tricuspid Insufficiency Treatment Market Insights Summary:
Regional Highlights:
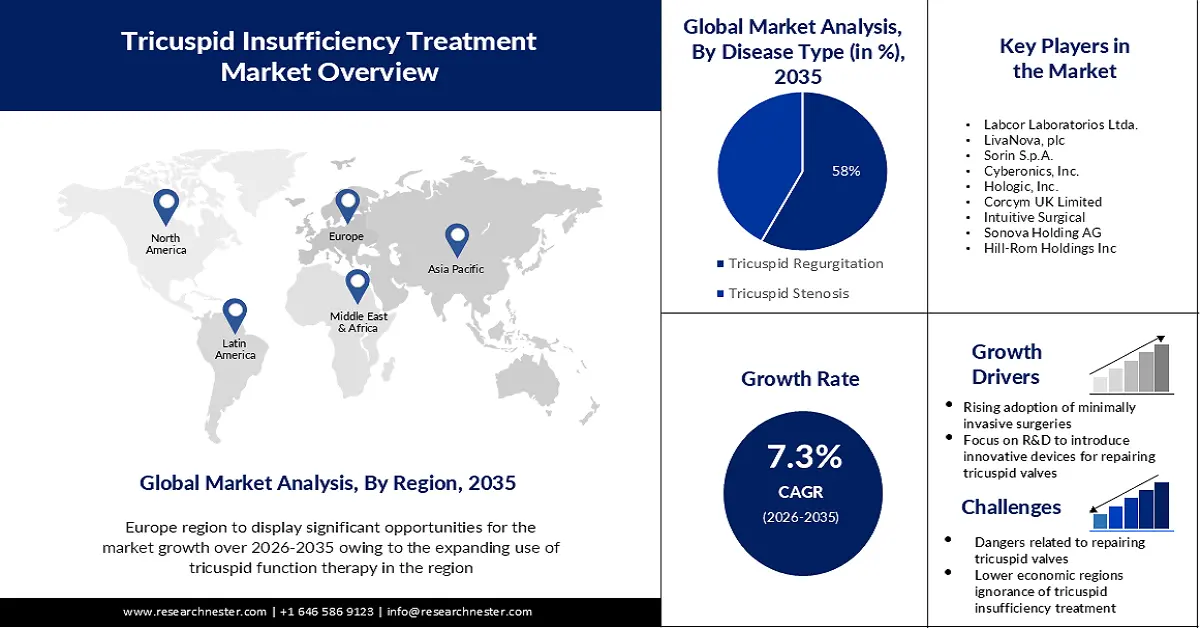
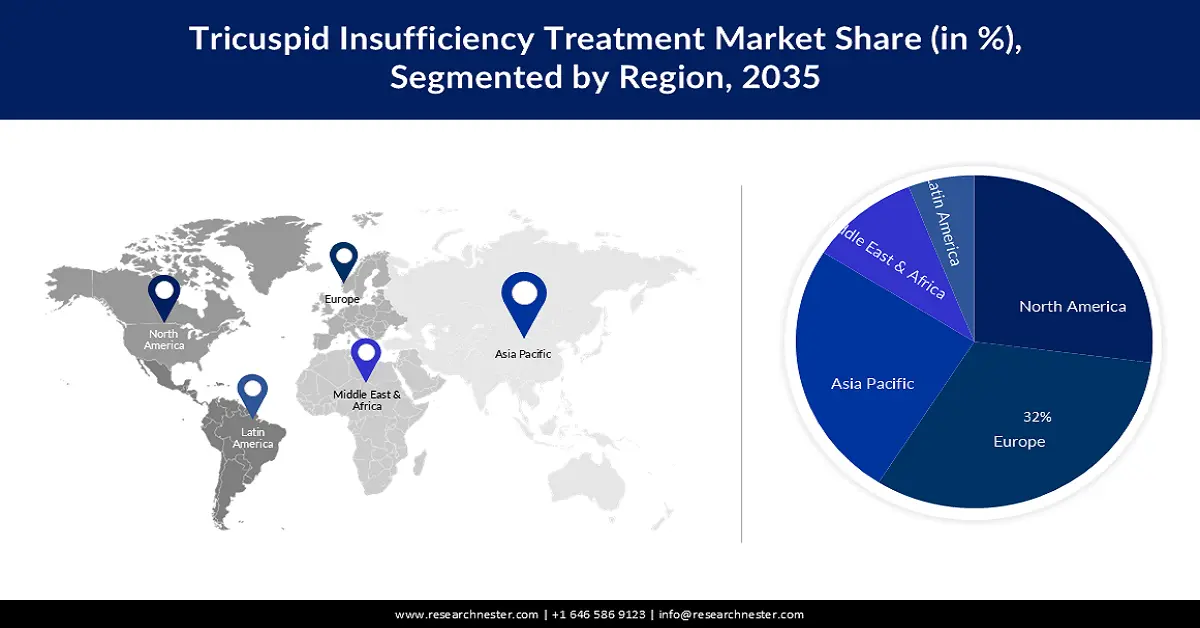
- By 2035, the Europe region in the tricuspid insufficiency treatment market is expected to secure a 32% share, sustained by the expanding use of tricuspid function therapy across major countries, especially Germany, and increasing investments in cardiac-care device research.
- By 2035, the North America region is projected to hold a 27% share, supported by rising awareness of valve repair or annuloplasty procedures and broader accessibility of advanced technologies.
Segment Insights:
- By 2035, the tricuspid regurgitation segment in the tricuspid insufficiency treatment market is projected to claim a 58% share, underpinned by technological advancements and the growing burden of CVDs amid higher alcohol and fast-food consumption.
- By 2035, the hospital segment is anticipated to hold about a 60% share, reinforced by expanding government and healthcare initiatives promoting timely treatment and increased adoption of advanced therapeutic technologies.
Key Growth Trends:
- Increase in Cardiovascular Surgery among the World's Population
- Focus on R&D to Introduce Innovative Devices for Repairing Tricuspid Valves
Major Challenges:
- Dangers Related to Repairing Tricuspid Valves
- Lower Economic Regions' Ignorance of Tricuspid Insufficiency Treatment
Key Players: Labcor Laboratorios Ltda., LivaNova, plc, Sorin S.p.A., Cyberonics, Inc., Hologic, Inc., Corcym UK Limited, Intuitive Surgical, Sonova Holding AG.
Global Tricuspid Insufficiency Treatment Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 778.33 million
- 2026 Market Size: USD 829.47 million
- Projected Market Size: USD 1.57 billion by 2035
- Growth Forecasts: 7.3%
Key Regional Dynamics:
- Largest Region: Europe (32% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, Germany, United Kingdom, China, Japan
- Emerging Countries: India, Brazil, South Korea, Italy, Canada
Last updated on : 19 November, 2025
Tricuspid Insufficiency Treatment Market - Growth Drivers and Challenges
Growth Drivers
- Increase in Cardiovascular Surgery among the World's Population- The world's population is shifting, and this has increased serious cardiovascular illnesses. It has been observed that approximately 697,000 deaths in the US in 2020 were related to cardiovascular illnesses. Globally, the prevalence of cardiovascular disorders is rising, which will increase market demand throughout the forecast period. Government programs to reduce the number of people who die from cardiovascular illnesses will also contribute to the market's expansion.
- Focus on R&D to Introduce Innovative Devices for Repairing Tricuspid Valves- The disclosure of invasive open-heart surgery post-surgical difficulties has forced cardiovascular manufacturers to launch novel approaches to tricuspid valve repair. This method will become the primary segment in the target market and further disrupt the current choice of operations. Heart doctors recommend these invasive products as essential inputs in clinical trials. For instance, individuals with severe tricuspid regurgitation have undergone clinical verification of a revolutionary transcatheter tricuspid valve replacement device (TTVR) made by Abbott under the brand name TriClip.
- Rising Adoption of Minimally Invasive or Robotic Surgeries- A further significant factor driving the industry's expansion is the growing popularity of minimally invasive procedures or robotic surgeries like annuloplasty. Manufacturers are concentrating on creating highly sophisticated annuloplasty rings as a result. An annuloplasty ring is used during tricuspid valve repair surgeries to improve long-term outcomes and decrease the likelihood of tricuspid regurgitation returning.
Challenges
- Dangers Related to Repairing Tricuspid Valves- The hazards of tricuspid valve repair are similar to those of any medical procedure; these concerns cannot be disregarded. Patients undergoing tricuspid valve repair may experience bleeding, infections, stroke, abnormal cardiac rhythms, blood clots, and failure of the repaired valve. In extreme circumstances, it might potentially be fatal. The market will be somewhat harmed by these post-surgery issues associated with tricuspid valve replacement. To reduce or completely remove the danger associated with the tricuspid valve repair operation, manufacturers of products for the repair must spend in research and development as well as clinical studies.
- Lower Economic Regions' Ignorance of Tricuspid Insufficiency Treatment
- The COVID-19 epidemic had a big impact on the industry. The stringent lockdowns and governmental limitations led to a drop in the number of heart surgeries.
Tricuspid Insufficiency Treatment Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
7.3% |
|
Base Year Market Size (2025) |
USD 778.33 million |
|
Forecast Year Market Size (2035) |
USD 1.57 billion |
|
Regional Scope |
|
Tricuspid Insufficiency Treatment Market Segmentation:
Disease Type Segment Analysis
The tricuspid regurgitation segment is attributed to hold 58% share of the global tricuspid insufficiency treatment market during the forecast period. The segment is expected to experience additional expansion due to a number of factors, including technological developments, an increase in the incidence of various CVDs as a result of a sedentary lifestyle adoption, and rising alcohol and fast food consumption. At least once a week, 2 out of 3 individuals eat fast food. The average monthly expenditure for fast food is USD 148. In addition, a lot of important businesses are concentrating on releasing new goods in order to increase their market share and obtain a competitive advantage.
End-User Segment Analysis
Tricuspid insufficiency treatment market from the hospital segment is projected to hold largest revenue share of about 60% during the forecast period. The segment's growth is further aided by the expanding initiatives of governments and healthcare facilities that emphasize the promotion of prompt examinations and treatments. Hospitals are typically able to provide better care and more advanced treatment options because they receive larger financing. The segment is expected to grow as a result of expanding hospital use of technologically advanced treatment methods and gadgets and growing investments in healthcare infrastructure. In addition to these, a more benevolent reimbursement procedure along with higher spending in research and development will spur additional growth. Globally, the pharmaceutical sector spent over 244 billion dollars on research and development in 2022. By contrast, in 2012, R&D spending reached a total of 137 billion dollars.
Our in-depth analysis of the global market includes the following segments:
|
Disease Type |
|
|
End-User |
|
|
Surgery Type |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Tricuspid Insufficiency Treatment Market - Regional Analysis
European Market Insights
Tricuspid insufficiency treatment market in the Europe industry is expected to account for largest revenue share of 32% by 2035 due to the expanding use of tricuspid function therapy in multiple nations in the area, primarily in Germany. Aside resulting in this, raising investments to make investigation and creation actions connected to cardiac health care gadgets are anticipated to spur market expansion in the area in the upcoming year. Presently, the EU has about 6 million new cases of CVD annually, while Europe as a whole has over 11 million instances. The condition affects around 49 million people in the EU, and it costs the economies of those countries USD 227 billion annually.
North American Market Insights
Tricuspid insufficiency treatment market in the North America region is attributed to hold the second largest share of about 27% by the end of 2035 owing to a number of variables, including patient knowledge of valve repair procedures or annuloplasty and the accessibility of high-tech products. The aging population is one of the primary drivers of the growth in the regional market. For example, according to data released by the Organisation for Economic Co-operation and Development (OECD) in 2022, the percentage of Americans 65 and older rose to 16.83% of the population in 2022. Furthermore, the gender difference in life expectancy is closing and the U.S. population is getting older, according to the Population Reference Bureau.
Tricuspid Insufficiency Treatment Market Players:
- Abbott Laboratories
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Labcor Laboratorios Ltda.
- LivaNova, plc
- Sorin S.p.A.
- Cyberonics, Inc.
- Hologic, Inc.
- Corcym UK Limited
- Intuitive Surgical
- Sonova Holding AG
- Hill-Rom Holdings Inc.
Recent Developments
- November 2019: LivaNova PLC, a announced it is ending its Caisson Transcatheter Mitral Valve Replacement (TMVR) program and will undertake a restructuring of its heart valve business to improve profitability and ensure business continuity. “The time has come to address the continued declines we have experienced in our heart valve business. We will restructure and simplify our heart valve manufacturing network, which will eliminate operational overlap between facilities and enable us to address new regulatory requirements,” said Damien McDonald, Chief Executive Officer of LivaNova. “As we evaluated these changes along with those in the structural heart market, we determined it was no longer viable to continue to invest in our TMVR program. As a result, we will close our Caisson TMVR operations.”
- September 2019: Abbott launched the first ever pivotal trial in the world to test new approach for repairing faulty tricuspid heart valves. The trial was aimed at evaluating the safety and effectiveness of the company’s TriClip transcatheter tricuspid valve repair system.
- Report ID: 3327
- Published Date: Nov 19, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




