Sezary Syndrome Market - Regional Analysis
North America Market Insights
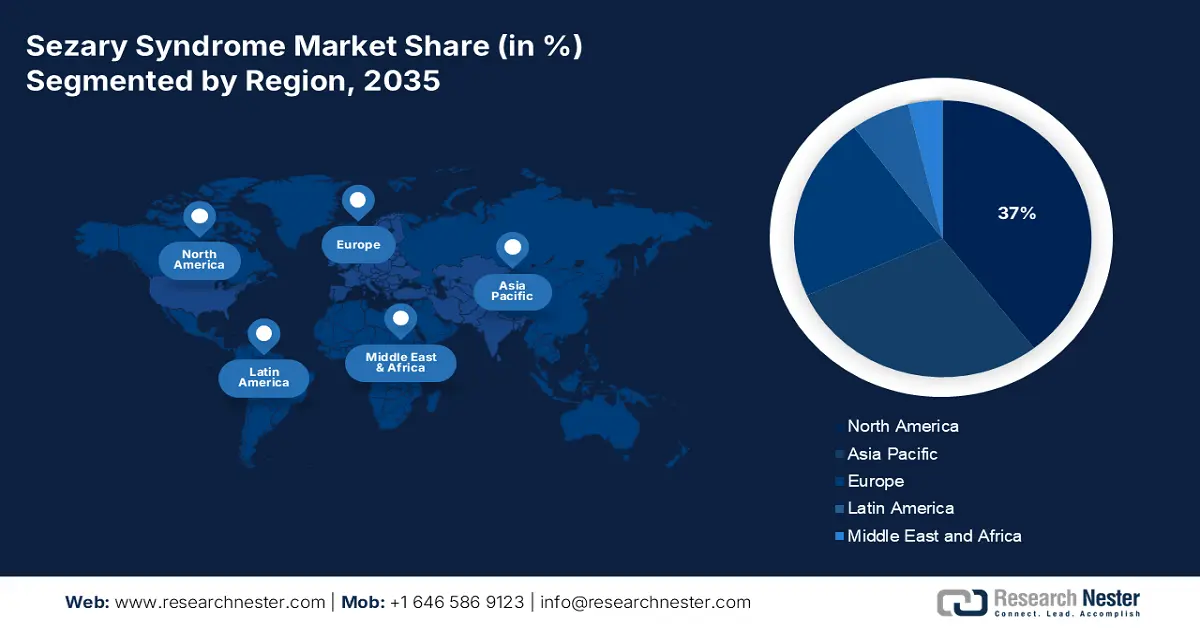
North America industry is predicted to dominate majority revenue share of 37% by 2035. Efforts to improve medical facilities and advance healthcare systems have made treatment options abundant in the region. Doctors in the United States are focused on relieving symptoms and improving quality of life when treating newly diagnosed stage III and stage IV mycosis fungoides, including Sézary syndrome, involving the use of Psoralen and ultraviolet A (PUVA) radiation therapy, as well as ultraviolet B radiation therapy.
Asia Pacific Market Insights
Approximately 25% of the Sezary syndrome market will come from Asia Pacific by 2035. There has been an increase in awareness regarding the disease in the region, a rise in the availability of drugs approved for approval, and the availability of advanced treatments and technologies. A total of 21 monoclonal antibody drugs have been approved by China (10 locally developed, 11 imported). Drugs of the monoclonal antibody class bind to specific cells or proteins in the body. In treating Sezary Syndrome, they stick to abnormal cells in the skin that cause the disease and reduce inflammation.