Sezary Syndrome Market Outlook:
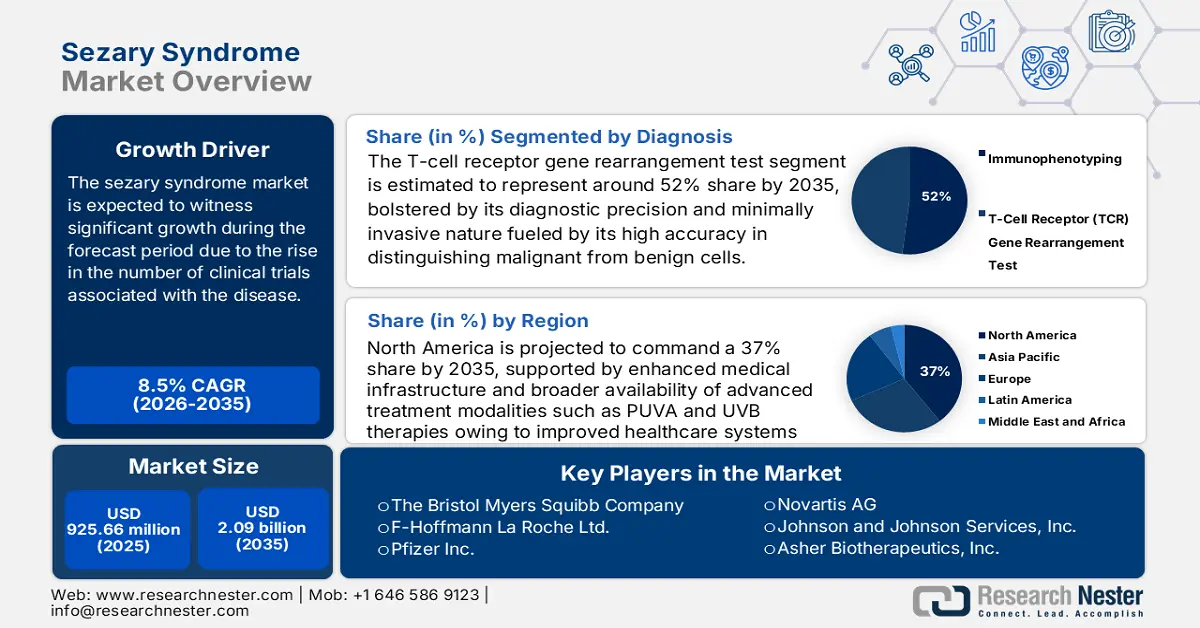
Sezary Syndrome Market size was over USD 925.66 million in 2025 and is anticipated to cross USD 2.09 billion by 2035, growing at more than 8.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of sezary syndrome is assessed at USD 996.47 million.
A sezary syndrome is a severe form of cutaneous T-cell lymphoma (CTCL), which is increasing in prevalence worldwide. Approximately six CTCL cases occur per million in the general population every year, age-adjusted. In most cases of Sézary syndrome, people live for at least five years after the disease develops. A rise in cases of this disorder increases demand for treatment and products associated with it.
The number of clinical trials and studies devoted to researching potential treatments for the syndrome is on the rise such as topical corticosteroid, topical mechlorethamine, and topical bexarotene, making it more likely that more effective treatments will be developed. Increasing awareness of the syndrome among medical professionals and improved funding for research into potential treatments have led to increased research efforts.
Key Sezary Syndrome Market Insights Summary:
Regional Highlights:
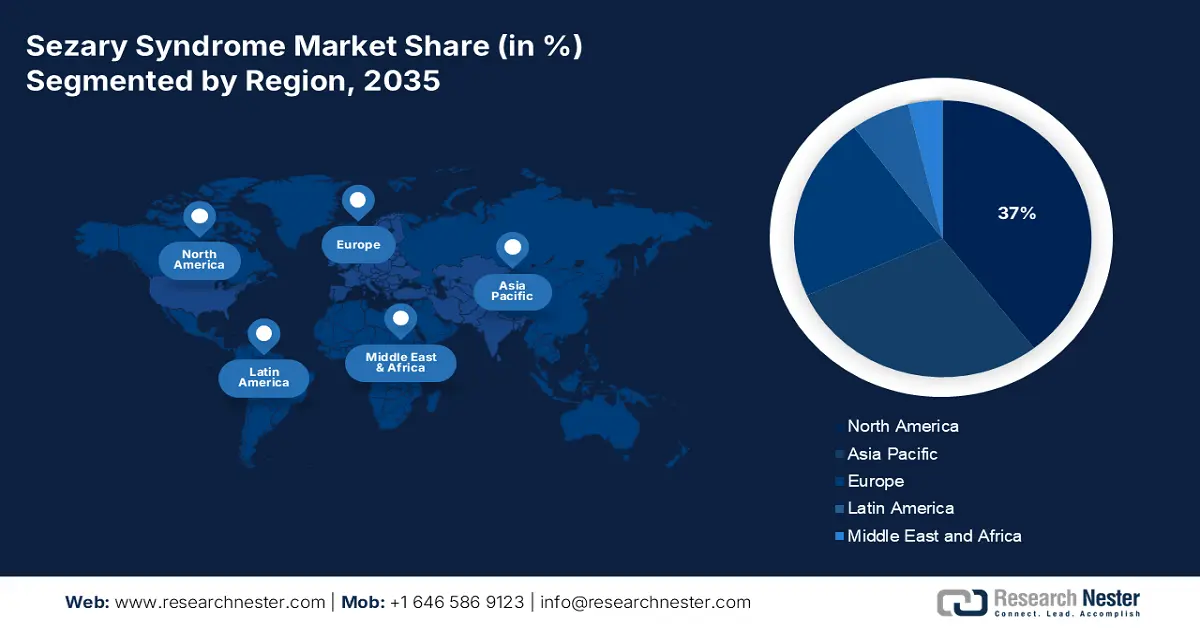
- North America is projected to command a 37% share of the sezary syndrome market by 2035, attributable to efforts to improve medical facilities and advance healthcare systems.
- Asia Pacific is expected to account for around 25% share by 2035, supported by rising disease awareness and increased availability of approved drugs and advanced treatments.
Segment Insights:
- T-cell receptor gene rearrangement test segment is projected to secure about 52% share by 2035, propelled by its high accuracy in detecting malignant cells and distinguishing benign from malignant cells.
- The radiation therapy segment is anticipated to hold nearly 46% share by 2035, underpinned by the increasing prevalence of Sezary syndrome, rising awareness of RT, and the adoption of advanced total skin electron beam (TSEB) radiation therapy.
Key Growth Trends:
- Rise In the Number of Clinical Trials Associated with The Disease
- Emergence of Novel Treatments for Sézary Syndrome
Major Challenges:
- Difficulty of diagnosing and treating syndrome
- Lack of awareness of the condition in emerging economies
Key Players: GSK plc, The Bristol Myers Squibb Company, F-Hoffmann La Roche Ltd., Pfizer Inc., Novartis AG, Johnson and Johnson Services, Inc., Asher Biotherapeutics, Inc., Be Biopharma, Eisai Co., Ltd., Seagen Inc.
Global Sezary Syndrome Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 925.66 million
- 2026 Market Size: USD 996.47 million
- Projected Market Size: USD 2.09 billion by 2035
- Growth Forecasts: 8.5%
Key Regional Dynamics:
- Largest Region:North America (37% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, Japan, United Kingdom
- Emerging Countries:India, South Korea, Brazil, Australia, Canada
Last updated on : 25 November, 2025
Sezary Syndrome Market - Growth Drivers and Challenges
Growth Drivers
-
Rise In the Number of Clinical Trials Associated with The Disease - University College London and Merck Sharp & Dohme LLC have been conducting Phase II clinical trials since January 2019. The study aims to assess the effectiveness of Pembrolizumab combined with radiotherapy for patients with relapsed or refractory mycosis fungoides/Sezary syndrome.
-
Emergence of Novel Treatments for Sézary Syndrome - An antibody therapy called mogamulizumab targets a protein called CC chemokine receptor (CCR4) for the treatment of Sézary syndrome. Skin-cell lymphoma cells commonly contain this protein. National Institute for Health and Care Excellence will assess whether the treatment is eligible for NHS funding.
- A Rise in Biomarker Research and Development - In the early 21st century, regulations in the field of biomarkers developed rapidly and are closely linked to the development of personalized medicine, which involves tailoring treatment based on the genetic and epigenetic characteristics of an individual. This has enabled a better understanding of Sezary Syndrome and the ability to diagnose and treat the condition more effectively.
Challenges
-
Difficulty of diagnosing and treating syndrome- Sezary Syndrome is a rare form of skin cancer that is often difficult to diagnose due to its vague symptoms. Additionally, treatments for Sezary Syndrome are often complicated, expensive, and not always effective, which can limit the market growth for treatments.
-
Lack of awareness of the condition in emerging economies
- Limited research and approved treatments available for the disease
Sezary Syndrome Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
8.5% |
|
Base Year Market Size (2025) |
USD 925.66 million |
|
Forecast Year Market Size (2035) |
USD 2.09 billion |
|
Regional Scope |
|
Sezary Syndrome Market Segmentation:
Diagnosis Segment Analysis
A market share of about 52% is estimated for the T-cell receptor gene rearrangement test segment in 2035. As a result of its high accuracy in detecting malignant cells and its ability to distinguish between benign and malignant cells, the TCR test is crucial in diagnosing and treating this condition. Additionally, the TCR gene rearrangement test is relatively quick and non-invasive, reducing the risk of potential side effects from more invasive tests.
Therapy Type Segment Analysis
Sezary syndrome market from the radiation therapy segment is expected to gain a significant share of about 46% by 2035 driven by the increasing prevalence of sezary syndrome, increasing awareness about RT, and the introduction of new technologies such as total skin electron beam (TSEB) radiation therapy. Radiation therapy using TSEB aims electrons at the whole body's skin to treat this condition. Additionally, radiation therapy has fewer side effects compared to other treatments and is associated with a higher cure rate.
Our in-depth analysis of the market includes the following segments:
|
Treatment Type |
|
|
Diagnosis |
|
|
Drug Class |
|
|
Therapy Type |
|
|
End User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Sezary Syndrome Market - Regional Analysis
North America Market Insights
North America industry is predicted to dominate majority revenue share of 37% by 2035. Efforts to improve medical facilities and advance healthcare systems have made treatment options abundant in the region. Doctors in the United States are focused on relieving symptoms and improving quality of life when treating newly diagnosed stage III and stage IV mycosis fungoides, including Sézary syndrome, involving the use of Psoralen and ultraviolet A (PUVA) radiation therapy, as well as ultraviolet B radiation therapy.
Asia Pacific Market Insights
Approximately 25% of the Sezary syndrome market will come from Asia Pacific by 2035. There has been an increase in awareness regarding the disease in the region, a rise in the availability of drugs approved for approval, and the availability of advanced treatments and technologies. A total of 21 monoclonal antibody drugs have been approved by China (10 locally developed, 11 imported). Drugs of the monoclonal antibody class bind to specific cells or proteins in the body. In treating Sezary Syndrome, they stick to abnormal cells in the skin that cause the disease and reduce inflammation.
Sezary Syndrome Market Players:
- GSK plc
- The Bristol Myers Squibb Company
- F-Hoffmann La Roche Ltd.
- Pfizer Inc.
- Novartis AG
- Johnson and Johnson Services, Inc.
- Asher Biotherapeutics, Inc.
- Be Biopharma
- Eisai Co., Ltd.
- Seagen Inc.
Recent Developments
- The Japan-based Eisai Co., Ltd. has received approval to market and manufacture Remitoro for the treatment of relapsed or refractory PTCL or CTCL. The drug Remitoro is considered a high-need drug.
- Seagen Inc. has announced new data for ADCETRIS will be presented at the 64th Annual Meeting and Exposition of the American Society of Hematology (ASH). An updated and interim efficacy and safety clinical trial study for ADCETRIS in classical Hodgkin lymphoma (cHL) and other CD30-expressing lymphomas as well as other rare cancers was presented in the abstracts, including five oral presentations.
- Report ID: 4996
- Published Date: Nov 25, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Sezary Syndrome Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




