PART 01
- Introduction
- Study Objective
- Scope of the Report
- Study Assumptions and Abbreviations
PART 02
- Research Methodology
- Secondary Research
- Primary Research
- Data Triangulation
- Client-Specific Requirements and Tailored Solutions
PART 03
- Executive Summary
- Competitive Landscape
- Competitive Intelligence
- Strategic Imperative
- Outcome: Actionable Insights
PART 04
- Global Economic Outlook
PART 05
- Regional Outlook
PART 06
- Global Industry Overview
- Global RNase Control Market Overview
- Market Value (USD Million), Current and Future
- Projections, 2024 2037
- Increment $ Opportunity Assessment, 2024 2037
- Year on Year Growth Forecast (%)
- Global RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- Global RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- Global RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Global RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
Cross Analysis of Product Type w.r.t. End-User
- North America RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- North America RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- North America RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- North America RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- North America RNase Control Market Segmentation Analysis (2024-2037)
- By Country
- US, Market Value (USD Million), CAGR, 2024-2037F
- Canada, Market Value (USD Million), CAGR, 2024-2037F
- By Country
Cross Analysis of Product Type w.r.t. End-User
- US RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- US RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- US RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- US RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- Canada RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- Canada RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- Canada RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Canada RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- Latin America RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Latin America RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- Latin America RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Latin America RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- Latin America RNase Control Market Segmentation Analysis (2024-2037)
- By Country
- Brazil, Market Value (USD Million), CAGR, 2024-2037F
- Argentina, Market Value (USD Million), CAGR, 2024-2037F
- Mexico, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Latin America, Market Value (USD Million), CAGR, 2024-2037F
- By Country
Cross Analysis of Product Type w.r.t. End-User
- Europe RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Europe RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- Europe RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Europe RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- Europe RNase Control Market Segmentation Analysis (2024-2037)
- By Country
- U.K., Market Value (USD Million), CAGR, 2024-2037F
- Germany, Market Value (USD Million), CAGR, 2024-2037F
- France, Market Value (USD Million), CAGR, 2024-2037F
- Italy, Market Value (USD Million), CAGR, 2024-2037F
- Spain, Market Value (USD Million), CAGR, 2024-2037F
- NORDIC, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Europe, Market Value (USD Million), CAGR, 2024-2037F
- By Country
- By Control Type
Cross Analysis of Product Type w.r.t. End-User
- UK RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- UK RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- UK RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- UK RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- By Control Type
- Germany RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- Germany RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Germany RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Germany RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- By Product Type
- France RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- France RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- France RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- France RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- By Control Type
- Italy RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Italy RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- Italy RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Italy RNase Control Market Segmentation Analysis (2024-2037)
- By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By End-User
- By Control Type
- Spain RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Spain RNase Control Market Segmentation Analysis (2024-2037)
- By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Product Type
- Spain RNase Control Market Segmentation Analysis (2024-2037)
- By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Application
- Spain RNase Control Market Segmentation Analysis (2024-2037) By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- NORDIC RNase Control Market Segmentation Analysis (2024-2037) By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- NORDIC RNase Control Market Segmentation Analysis (2024-2037) By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- NORDIC RNase Control Market Segmentation Analysis (2024-2037) By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- NORDIC RNase Control Market Segmentation Analysis (2024-2037) By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- Rest of Europe RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Europe RNase Control Market Segmentation Analysis (2024-2037) By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Europe RNase Control Market Segmentation Analysis (2024-2037) By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Europe RNase Control Market Segmentation Analysis (2024-2037) By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
- Asia Pacific RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Asia Pacific RNase Control Market Segmentation Analysis (2024-2037) By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Asia Pacific RNase Control Market Segmentation Analysis (2024-2037) By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Asia Pacific RNase Control Market Segmentation Analysis (2024-2037) By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Asia Pacific RNase Control Market Segmentation Analysis (2024-2037) By Country
- China, Market Value (USD Million), CAGR, 2024-2037F
- Japan, Market Value (USD Million), CAGR, 2024-2037F
- India, Market Value (USD Million), CAGR, 2024-2037F
- Indonesia, Market Value (USD Million), CAGR, 2024-2037F
- Singapore, Market Value (USD Million), CAGR, 2024-2037F
- Malaysia, Market Value (USD Million), CAGR, 2024-2037F
- Thailand, Market Value (USD Million), CAGR, 2024-2037F
- Vietnam, Market Value (USD Million), CAGR, 2024-2037F
- South Korea, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Asia Pacific, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
Cross Analysis of Product Type w.r.t. End-User
- Middle East & Africa RNase Control Market Segmentation Analysis (2024-2037)
- By Control Type
- RNase-Free Reagents and Kits, Market Value (USD Million), CAGR, 2024-2037F
- RNase Removal Solutions, Market Value (USD Million), CAGR, 2024-2037F
- RNase Inhibitors, Market Value (USD Million), CAGR, 2024-2037F
- Decontamination Sprays and Wipes, Market Value (USD Million), CAGR, 2024-2037F
- Middle East & Africa RNase Control Market Segmentation Analysis (2024-2037) By Product Type
- RNase A, Market Value (USD Million), CAGR, 2024-2037F
- RNase H, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Middle East & Africa RNase Control Market Segmentation Analysis (2024-2037) By Application
- RNA Extraction and Purification, Market Value (USD Million), CAGR, 2024-2037F
- RT-PCR and qPCR, Market Value (USD Million), CAGR, 2024-2037F
- RNA Sequencing, Market Value (USD Million), CAGR, 2024-2037F
- Gene Expression Analysis, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Middle East & Africa RNase Control Market Segmentation Analysis (2024-2037) By End-User
- Academic and Research Institutes, Market Value (USD Million), CAGR, 2024-2037F
- Pharmaceutical and Biotechnology Companies, Market Value (USD Million), CAGR, 2024-2037F
- Diagnosis Laboratories, Market Value (USD Million), CAGR, 2024-2037F
- Others, Market Value (USD Million), CAGR, 2024-2037F
- Middle East & Africa RNase Control Market Segmentation Analysis (2024-2037) By Country
- GCC, Market Value (USD Million), CAGR, 2024-2037F
- Israel, Market Value (USD Million), CAGR, 2024-2037F
- South Africa, Market Value (USD Million), CAGR, 2024-2037F
- Rest of Middle East & Africa, Market Value (USD Million), CAGR, 2024-2037F
- By Control Type
Cross Analysis of Product Type w.r.t. End-User
PART 07
- Industry Supply Chain Analysis
PART 08
- Market Dynamics
PART 09
- Government Regulation: How they would Aid the Business?
PART 10
- Competitive Landscape
- Company Market Share
- Business Profile of Key Enterprises
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Qiagen N.V.
- New England Biolabs.
- Roche Diagnostics
- Illumina, Inc.
- Beyotime
- Bio Rad Laboratories, Inc.
- Others
PART 11
- Product Analysis
PART 12
- Patent Analysis
PART 13
- SWOT Analysis
PART 14
- Price Benchmarking
PART 15
- End-User Analysis
PART 16
- PEST Analysis
PART 17
- Industry Risk Assessment
RNase Control Market Outlook:
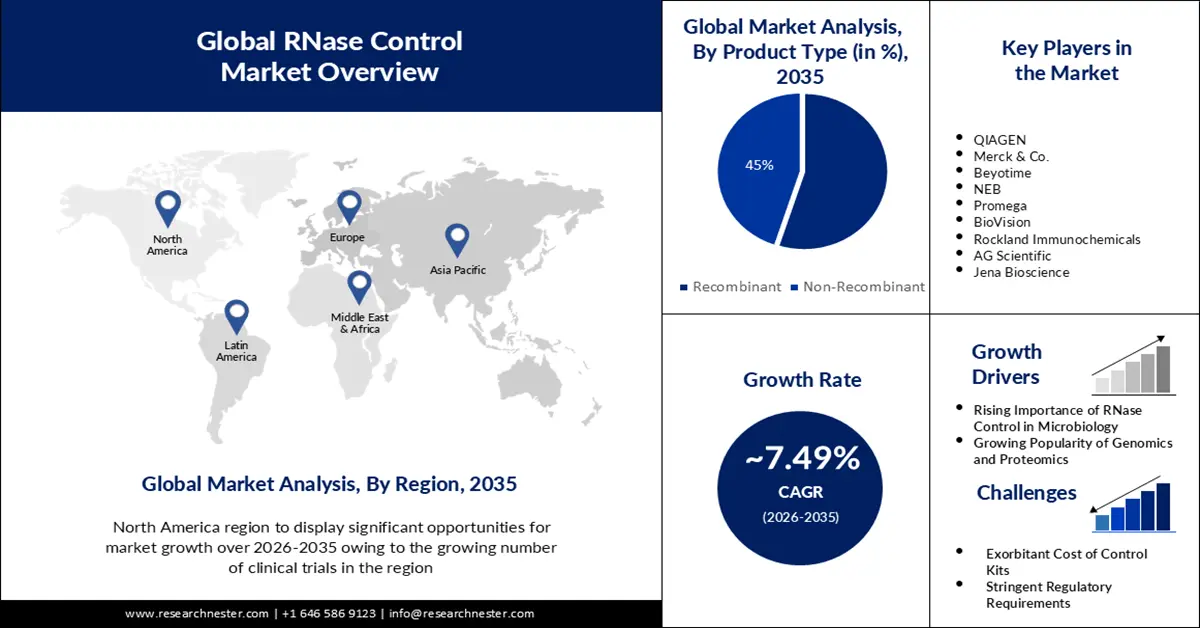
RNase Control Market size was valued at USD 190.79 Million in 2025 and is expected to reach USD 392.86 Million by 2035, registering around 7.49% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of RNase control is evaluated at USD 203.65 Million.
Some key factors driving the growth of the RNase control market include an increase in patients with genetic diseases like sickle cell disease, cancer, and Huntington's disease-like 2 (HDL2).
Other factors include introducing novel detection tests and numerous genetic research initiatives. A National Library of Medicine study from February 2020 states that epidemiological data showed 71.9% of 6,172 distinct uncommon illnesses were hereditary. Because genetic illnesses are becoming more common, there is a greater demand for gene treatments and gene-based research, which depends heavily on RNA surveillance.
Key RNase Control Market Insights Summary:
Regional Highlights:
- North America region is anticipated to secure a considerable revenue share by 2035 in the RNase control market, propelled by advancements in technology and enhanced collaboration in the gene sector.
- Europe region is expected to capture substantial share by 2035, owing to its critical role in molecular biology and prevention of RNA degradation during key processes.
Segment Insights:
- RNase A segment is projected to account for over 7.56% share by 2035 in the RNase control market, owing to its widespread use in research and targeting by most RNase inhibitors.
- Pharmaceutical & biotechnology companies segment is expected to hold a significant share by 2035, driven by the need for precise gene expression analysis in target validation, biomarker identification, and efficacy evaluation.
Key Growth Trends:
- Escalating the genomics field
- Positive impact of COVID 19 pandemic
Major Challenges:
- High cost of control kits
- Lack of skilled laborers are estimated to hamper the market growth in the forecast period
Key Players: Roche Diagnostics Corporation, Illumina Inc., Bio-Rad Laboratories, Inc., Meridian Bioscience Inc., Takara Bio Inc., Genomics Ltd.
Global RNase Control Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 190.79 Million
- 2026 Market Size: USD 203.65 Million
- Projected Market Size: USD 392.86 Million by 2035
- Growth Forecasts: 7.49%
Key Regional Dynamics:
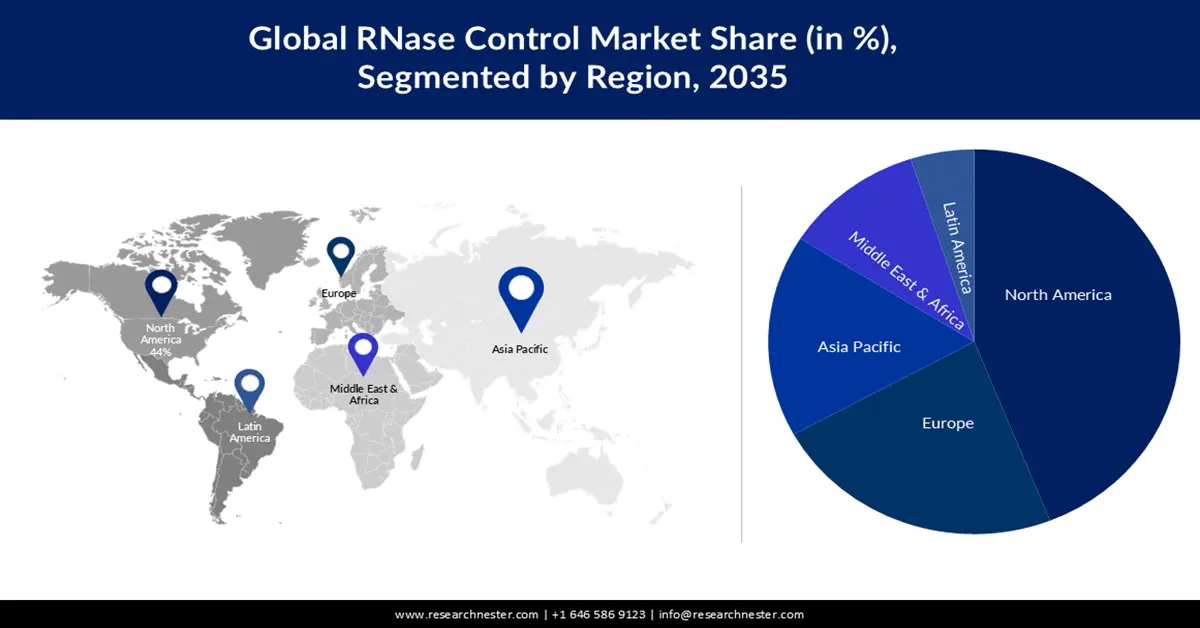
- Largest Region: North America (considerable share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, Germany, Japan, China, United Kingdom
- Emerging Countries: India, Brazil, South Korea, Australia, Canada
Last updated on : 26 November, 2025
RNase Control Market - Growth Drivers and Challenges
Growth Drivers
- Escalating the genomics field - In a rapidly growing field of proteomics and genomics, the use of RNase Control will be needed, which will increase demand worldwide. The modern multidisciplinary research collaboration's methodology and the usage of RNase Control will be necessary due to the expanding field of molecular biology research.
- Positive impact of COVID-19 pandemic - Owing to the high penetration of RNase Control in the identification of nucleic acids from SARS-CoV-2 the market saw a positive impact over the covid-19 pandemic period. The COVID-19 pandemic had a slightly positive impact on the RNase control market due to the use of RNase Control in detecting nucleic acids from SARS-CoV-2.
This increased use of these products is due to the increase in hospital and laboratory visits for testing purposes. In addition, several government initiatives have been taken to reduce the prices for RTPCR tests in emerging countries eventually leading to increased product use.
Challenges
- High cost of control kits - Developing and manufacturing RNase Control kits consists of standard quality control measures and ongoing research and development. These factors contribute to the high cost of the kit which is expected to hinder the RNase control market growth in the upcoming period.
- Lack of skilled laborers are estimated to hamper the market growth in the forecast period
- Complexities associated are expected to obstruct the market growth in the forecast period
RNase Control Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
7.49% |
|
Base Year Market Size (2025) |
USD 190.79 Million |
|
Forecast Year Market Size (2035) |
USD 392.86 Million |
|
Regional Scope |
|
RNase Control Market Segmentation:
Product Type Segment Analysis
RNase A segment is expected to dominate RNase control market share of over 7.56% by 2035. In research, RNase A is often used, and this type of RNase is also targeted by most of the RNase inhibitors on the market. Because ribonuclease A is found in high quantities in the pancreas of mammals and some reptiles, it's a member of the Pancreatic Ribonuclease Family.
End-User Segment Analysis
By the end of 2035, pharmaceutical & biotechnology companies segment is predicted to capture significant RNase control market share. Precise gene expression analysis is required for target validation, identification of biomarkers, and the evaluation of efficacy in pharmaceutical and biotechnology companies engaged in discovery, development, or quality control.
Maintaining RNase Control is crucial for guaranteeing the precision of data and facilitating well-informed choices in the development of pharmaceuticals. Organizations striving to bring their research outcomes or diagnostic instruments to market depend on precise and consistent data to confirm the effectiveness of their products.
Our in-depth analysis of the global RNase control market includes the following segments:
|
Control Type |
|
|
Product Type |
|
|
Application |
|
|
End-User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
RNase Control Market - Regional Analysis
North American Market Insights
In RNase control market, North America region is projected to account for considerable revenue share by the end of 2035. The robust growth of technology and increased cooperation in the gene sector are mainly to blame for this increase. Due to the prevention of degradation or contamination of RNA, these products have been widely applied in this area.
In addition, market growth in North America is driven by increased R&D and investment in the gene sector. For example, in October 2021, the US Food and Drug Administration, the National Institutes of Health, pharmaceutical companies, and five not-for-profit organizations joined forces to increase the research and development of gene therapy, which benefits approximately 30.0 million Americans.
The rising incidence of diseases, particularly genetic diseases, has led to extensive use of techniques such as polymerase chain reaction (PCR), which relies on RNase Control. A significant driver of the US RNase control market growth is this increased use in genetic research and disease diagnosis. The prevalence of reported genetic disorders in children aged 0 to 17 years in the United States was approximately 0.039, representing approximately 2.8 million children, according to a study based on a combined national survey of children's health conducted in 2016.
Canada is addressing the problem of RNase contamination by using specific decontaminants, strictly adhering to laboratory protocols, and focusing on preventing contamination from a variety of sources. By minimizing the impact of RNase contamination in laboratory settings, these measures aim to ensure the integrity of research and diagnostic procedures.
European Market Insights
Europe region in RNase control market is likely to capture substantial revenue share by 2035. Its important role in molecular biology, especially the prevention of RNA degradation during cDNA synthesis, RT-PCR, RNA purification, and other processes, is leading to a growing need for RNase Control across Europe. This is important because RNase contamination can be difficult to detect and may lead to the destruction of studies.
The growth of the RNase control market in the United Kingdom is supported by demand from all application sectors, as well as regulations that are favourable to regulators. In addition, the dynamics of demand for RNase Control are influenced by a broad range of options available from market players.
In Germany, a number of health compliance regulations apply to cooperation between the medical sector and healthcare professionals. This Regulation shall also apply to all cooperation and business activities in the digital health sector, indicating a regulatory framework that influences the adoption and use of healthcare-related products and technologies, including the control of RNase.
The increasing incidence of diseases, including genetic disease, has led to extensive use of methods for controlling RNase in France despite the disruption caused by the COVID-19 pandemic. The market for RNase Control in the country is likely to be driven by this trend.
RNase Control Market Players:
- Thermo Fisher Scientific Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Merck KGaA
- Qiagen N.V.
- New England Biolabs
- Roche Diagnostics Corporation
- Illumina Inc.
- Bio-Rad Laboratories, Inc.
- Meridian Bioscience Inc.
- Takara Bio Inc.
- Genomics Ltd
Recent Developments
- Merck and the Acceleration Consortium, based at the University of Toronto, Canada, have jointly announced the availability of their AI-driven experimentation planner, Bayesian Back End, open source on GitHub with an unrestricted Apache 2.0 license.
- Roche has signed a definitive agreement to acquire certain components of the LumiraDx group, which is related to novel point of care technologies developed by LumiraDx. Transformation testing at the point of care will be possible with the addition of LumiraDx technology to the diagnostic portfolio. A highly flexible platform has been developed by LumiraDx, which is capable of providing excellent performance in multiple disease areas and technology.
- Report ID: 5512
- Published Date: Nov 26, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
RNase Control Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




