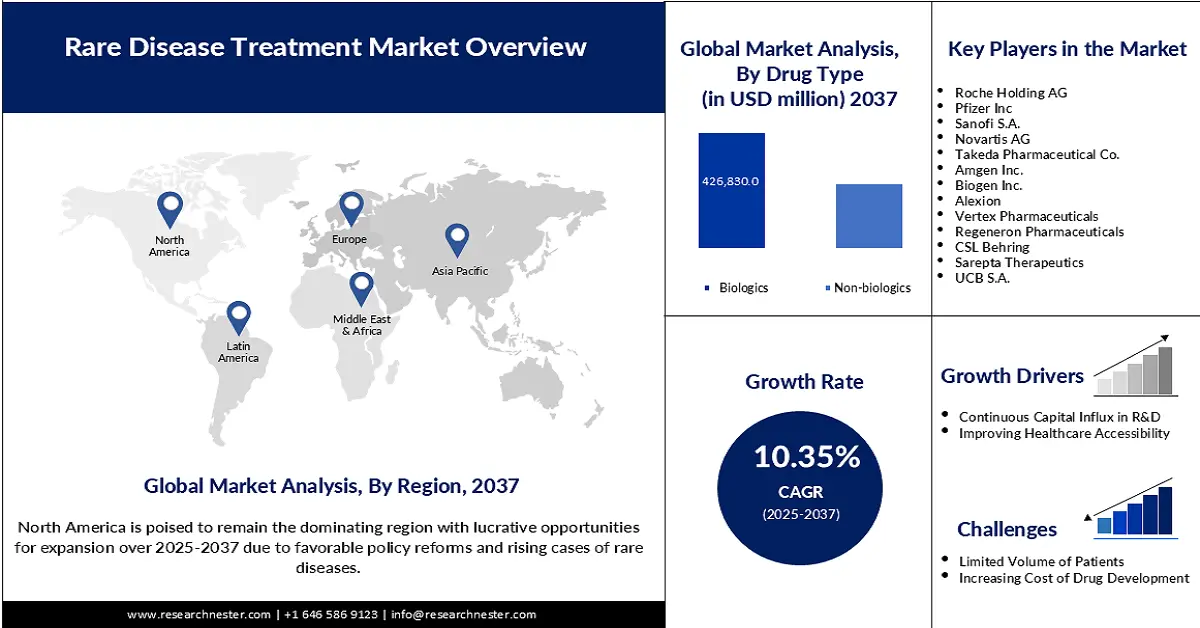
Rare Disease Treatment Market Outlook:
Rare Disease Treatment Market size was USD 232.2 billion in 2024 and is estimated to reach USD 792.8 billion by the end of 2037, expanding at a CAGR of 10.35% during the forecast period, i.e., 2025-2037. In 2025, the industry size of rare disease treatment is assessed at USD 243.1 billion.
The global rare disease treatment market is characterized by a limited yet diverse pool of patients, with more than 300 million people worldwide affected by such ailments in 2024, as reported by the Global Rare Disease Commission (GRDC). In key landscapes, including the U.S., Japan, and Europe, the target demography is quite small, but the cumulative epidemiology continues to grow due to improving diagnostic capacity and heritability of genetic mutations. As a result, approximately 70% of these illnesses appear among children. The wide range of these conditions also fuels demand in this sector, where more than 7,000 types of rare diseases were identified around the globe till 2024, with a potential to exceed 10,000 and 300 new rare genetic disease descriptions being added every year to the principal knowledge bases.
Further, indicating the financial overview of payers’ pricing in the market, the 2022 Institute for Clinical and Economic Review (ICER) unveiled that an annual orphan drug price of USD 100.0 thousand for a limited patient population of 10.0 thousand with these illnesses can earn a USD 1.0 billion yearly revenue. The report further mentioned that the overall cost of treating these conditions per individual often surpasses USD 1.0 million every year, owing to the high pricing of certified orphan drugs and gene & cell therapies. These figures indicate inflation in product and service costs for end-users and consumers, along with the lucrative opportunities from premium-priced pharmaceutical commodities.