Rabeprazole Sodium Market Outlook:
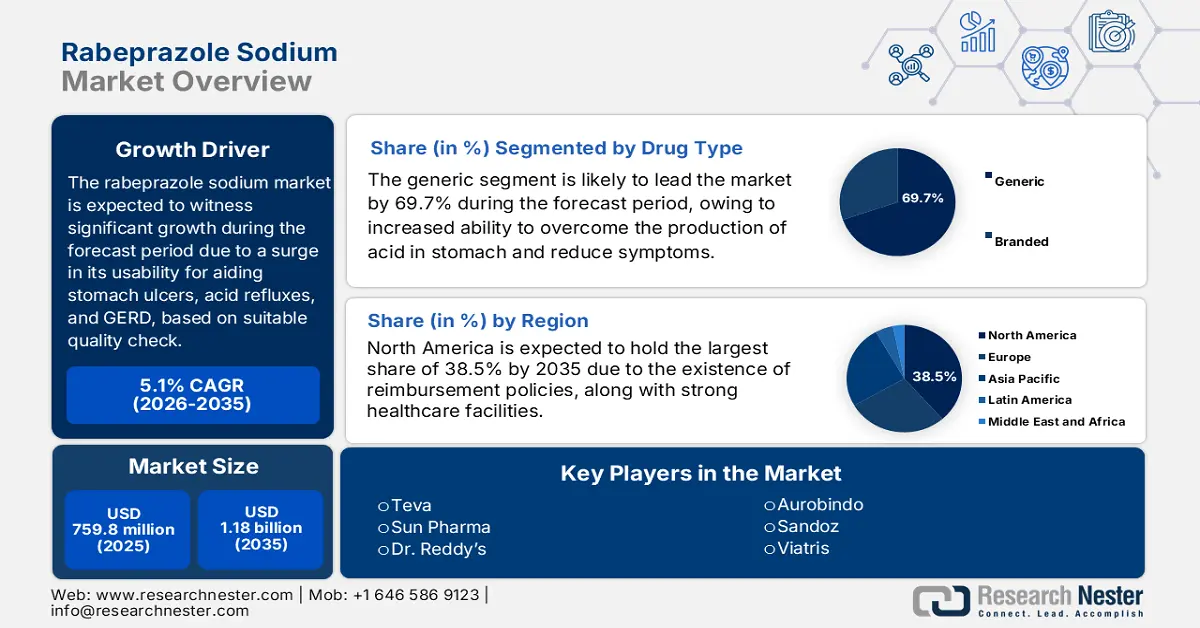
Rabeprazole Sodium Market size was over USD 759.8 million in 2025 and is estimated to reach USD 1.18 billion by the end of 2035, exhibiting a CAGR of 5.1% during the forecast period, i.e., 2026-2035. In 2026, the industry size of rabeprazole sodium is estimated at USD 798.5 million.
The international market is gaining increased traction, owing to its application for effectively aiding Zollinger-Ellison syndrome, gastroesophageal reflux disease (GERD), and duodenal ulcers. According to an article published by NLM in May 2022, for the past 6 years, the incidence of GERD has been 783.9 million cases. In addition, prevalent cases surged by 77.5%, which is followed by an increase in incidents by 74.7%, and years of life lived with disability (YLDs) by 77.1%. Therefore, the continuous upsurge of the disease, there is a huge demand for different kinds of rabprazole sodium drugs across both developed and developing nations, thus bolstering the overall market globally.
Moreover, the aspect of quality maintenance is also essential to ensure the immediate release of rabeprazole sodium drugs in the marketplace. In this regard, the February 2021 article indicated that the stability for the pharmaceutical-based dosage form is considered for a year, and constitutes an increased dissolution rate, which is more than 90% within half an hour. Besides, the inhibition of proton pump inhibitors, during the quality assurance process, successfully block K+-ATPase and H+-ATPase. PPIs are able to implement this by effectively passing through the parietal basement membrane, and accumulate in the in the secretory canaliculus where they tend to get activated with a pH scale of more than 4.0, thus suitable for the overall market, particularly during the manufacturing process.
Key Rabeprazole Sodium Market Insights Summary:
Regional Insights:
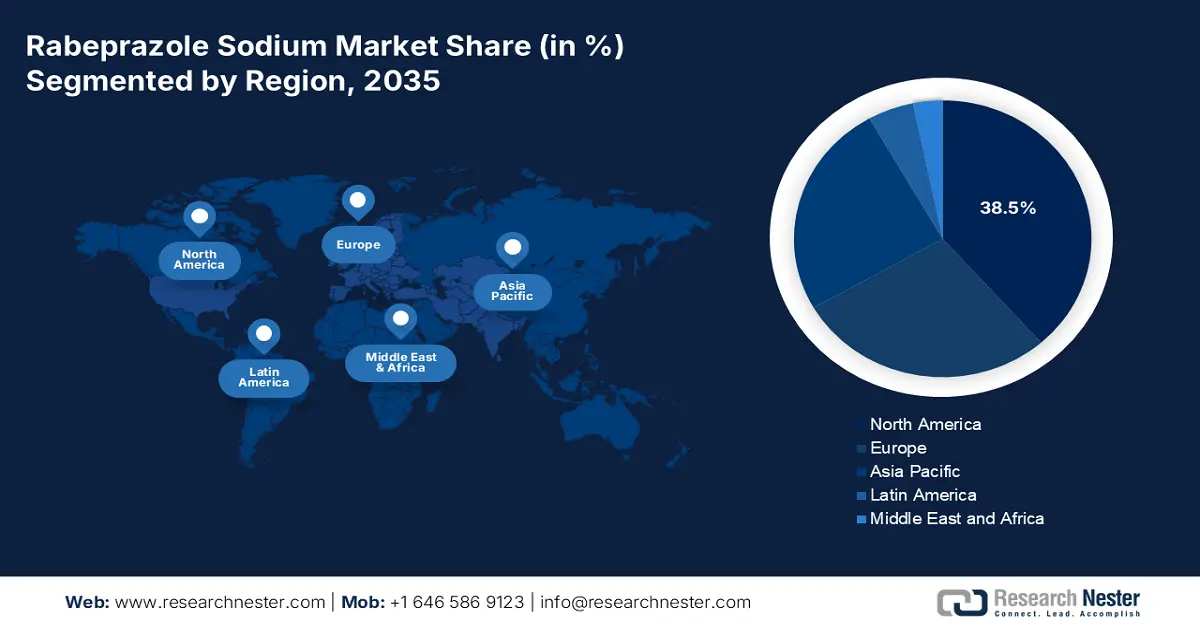
- Across 2026–2035, North America in the rabeprazole sodium market is anticipated to capture a 38.5% share by 2035, supported by escalating healthcare expenditure and structured Medicare and Medicaid reimbursement policies.
- By 2035, Asia Pacific is forecast to secure a 25.1% share, uplifted by government-led generic drug initiatives, expanding OTC availability, and the rise of localized API manufacturing.
Segment Insights:
- By 2035, the generic segment in the rabeprazole sodium market is expected to command a 69.7% share, stimulated by its effectiveness in reducing gastric acid, alleviating GERD and ulcer symptoms, and preventing esophageal damage.
- Through 2026–2035, the tablets segment is projected to reach a 59.2% share by 2035, supported by its efficient dissolution in the small intestine to optimize proton pump inhibition.
Key Growth Trends:
- Increase in generic drug approvals
- Expansion in healthcare facilities
Major Challenges:
- Therapeutic-based substitution pressure
- Increase in sustainability cost compliance
Key Players: Teva Pharmaceutical (Israel), Sun Pharmaceutical (India), Dr. Reddy’s Laboratories (India), Sandoz (Novartis) (Switzerland), Mylan (Viatris) (USA), Aurobindo Pharma (India), Lupin Limited (India), Hikma Pharmaceuticals (UK), Cipla (India), Fresenius Kabi (Germany), Amneal Pharmaceuticals (USA), Stada Arzneimittel (Germany), Aspen Pharmacare (South Africa), Krka Group (Slovenia), Alkaloid AD (North Macedonia)
Global Rabeprazole Sodium Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 759.8 million
- 2026 Market Size: USD 798.5 million
- Projected Market Size: USD 1.18 billion by 2035
- Growth Forecasts: 5.1% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (38.5% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, India, Japan, Germany
- Emerging Countries: Brazil, South Korea, Indonesia, Mexico, Turkey
Last updated on : 19 August, 2025
Rabeprazole Sodium Market - Growth Drivers and Challenges
Growth Drivers
- Increase in generic drug approvals: There has been a huge growth opportunity for the market, owing to the existence of generic drugs. These are essential for cost-effective healthcare accessibility, and advertising market competition. According to the October 2024 U.S. FDA report, an estimated savings from generic medication clearance accounts for a valuation of USD 16.6 billion, of which USD 1.7 billion caters to first-generic savings as of 2021, while USD 18.9 billion, of which USD 5.2 billion caters to first-generic savings as of the financial year 2022. Therefore, with such savings through administrative approvals, the market is poised to grow in the future.
- Expansion in healthcare facilities: This growth driver is essential for meeting patient demands, optimizing operational efficiency, and improving care quality. For instance, the May 2023 NLM article has indicated that the healthcare system in India is diversified and comprises complex private and public sectors, providing medical services to 1.4 billion population. In addition, the presence of the Ayushman Bharat scheme, as a health insurance program, initiated a coverage of INR 5 lakhs (USD 5,704) per family for the requirement of secondary and tertiary care services, thereby suitable for the market internationally.
- Transition towards online pharmacy: There has been a sudden shift towards to the online mode of pharmacy, owing to digitalization and innovation, thus denoting a prolific opportunity for the rabeprazole sodium market. As stated in the May 2024 NLM article, the digital pharmacy market is gradually growing a rate of 14.4%, and is expected to reach a market volume of USD 35.3 billion by the end of 2026. This has been readily successful with the digital technology adoption that tends to improve flexibility, efficiency, and productivity of healthcare systems, thereby creating a positive outlook for the market’s upliftment.
GORD Incidences Driving the Rabeprazole Sodium Market
GORD Cases Among the Adult Population Globally (2023)
|
Countries |
Percentage Range |
|
U.S. |
18.1 to 27.8 |
|
Middle East |
8.7 to 33.1 |
|
Europe |
8.8 to 25.9 |
|
South America |
23.0 |
|
Australia |
11.6 |
|
East Asia |
3 to 8 |
Source: Oxford Academic 2023
Safety and Healing Effectiveness of Anaprazole and Rabeprazole
Anaprazole and Rabeprazole Comparative Analysis (2021)
|
Factors |
Rabeprazole 10 mg |
Anaprazole 20 mg |
Anaprazole 40 mg |
|
Ulcer Healing Rate - FAS |
88.0% |
85.1% |
87.5% |
|
Ulcer Healing Rate - PPS |
88.9% |
86.0% |
90.9% |
|
Difference vs Rabeprazole 10 mg - FAS |
- |
2.9% (95% CI: 16.5 to 10.7) |
0.5% (95% CI: 13.5 to 12.5) |
|
Ulcer Healing Rate - FAS |
72.0% |
70.2% |
77.1% |
|
Ulcer Healing Rate - PPS |
75.6% |
72.1% |
79.5% |
|
Difference vs Rabeprazole 10 mg - FAS |
- |
-1.8% (95% CI: 19.8 to 16.3) |
+5.1% (95% CI: 12.2 to 22.3) |
|
Complete Symptom Relief |
More than 90% patients achieved relief |
More than 90% patients achieved relief |
More than 90% patients achieved relief |
Sources: Frontiers Organization, July 2021
Challenges
- Therapeutic-based substitution pressure: The presence of healthcare systems across different nations are vigorously marketing therapeutic substitution to low-cost PPIs, such as omeprazole. For instance, the NHS in the UK has readily prescribed this specific drug, which has resulted in the reduction of rabeprazole prescriptions. Likewise, the PMPRB in Canada has effectively enforced stringent price controls, which have increased inflation, along with research and development (R&D) expenses. These developments reflect payer prioritization in cost containment over product comparison, which has caused a hindrance in the rabeprazole sodium market globally.
- Increase in sustainability cost compliance: The sustainability aspect of the pharmaceutical industry has readily unveiled the newest cost pressures for manufacturers in the rabeprazole sodium market. For instance, the latest supply chain facility in Germany requires diligence-based legal actions, along with expensive audits based on social and environmental impacts. Besides, Sandoz has displayed sustainability as the competitive benefit, but small-scale organizations are witnessing existential risks from increased compliance expenses, which is negatively impacting the market.
Rabeprazole Sodium Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
5.1% |
|
Base Year Market Size (2025) |
USD 759.8 million |
|
Forecast Year Market Size (2035) |
USD 1.18 billion |
|
Regional Scope |
|
Rabeprazole Sodium Market Segmentation:
Drug Type Segment Analysis
The generic segment in the rabeprazole sodium market is projected to hold the largest share of 69.7% by the end of 2035. The segment’s growth is highly driven by its ability to combat the acid production in the stomach, diminish symptoms, and prevent injury to the esophagus among patients affected by GERD or ulcers. According to the April 2023 Oxford Academic report, for the past 10 years, GORD has represented 13.2% of the latest general medical practice, particularly in Australia, and has been ranked as the top 11 best managed conditions. Besides, utilizing a 6-month over-the-counter twice-daily generic omeprazole expense of USD 204 (USD 34/month), the PPI therapy option was derived as the least expensive, thereby suitable for the segment’s growth.
Dosage Form Segment Analysis
The tablets segment in the rabeprazole sodium market is expected to account for the second-largest share of 59.2% by the end of the forecast period. Rabeprezole sodium in tablet form is suitable for bypassing stomach acid and effectively gets dissolved in the small intestine, thus ensuring optimized proton pump inhibition. As per an article published by the Frontiers Organization in July 2021, 10 mg of rabeprazole tablets is highly recommended for almost 4 weeks, which is suitable for the segment’s growth. Besides, the April 2021 NLM article denoted that the success rate of a drug candidate usually ranges between 10% to 20%, thus denoting an optimistic outlook for rabeprazole sodium tablets.
End user Segment Analysis
The hospitals segment in the rabeprazole sodium market is predicted to hold the third-largest share of 50.7% during the projected period. The segment’s development is effectively propelled by bulk procurement benefits, as well as an increase in inpatient GRED treatment. Besides, PPI-based prescriptions stem from hospitals, which are readily supported by administrative clinical policies, particularly for acid-based diseases. Meanwhile, the generic implementation of rabeprazole sodium in hospitals tends to diminish expenses in comparison to branded medicated products, further escalating the rabeprazole sodium market penetration globally.
Our in-depth analysis of the rabeprazole sodium market includes the following segments:
|
Segment |
Subsegments |
|
Drug Type |
|
|
Dosage Form |
|
|
End user |
|
|
Application |
|
|
Distribution Channel |
|
|
Age |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Rabeprazole Sodium Market - Regional Analysis
North America Market Insights
North America in the rabeprazole sodium market is projected to be the dominant region by garnering the highest share of 38.5% by the end of 2035. The market’s growth in the region is effectively fueled by the existence of Medicare and Medicaid reimbursement policies, along with an increase in healthcare expenditure. For instance, as stated in the March 2023 JHEOR Organization report, the overall healthcare spending for GERD is USD 6,955, USD 8,755 for NDBE, USD 9,675 for IND, USD 12,241 for LGD, USD 24,329 for HGD, and USD 146,319 for EAC. All these denote the health expenses based on different symptoms of BE-related neoplasia (BERN) and Barrett’s esophagus (BE), which is positively uplifting the overall market demand in the region.
The rabeprazole sodium market in the U.S. is significantly growing due to the existence of standard sodium-based drugs to aid patients suffering from stomach acid diseases. For instance, 50 gm or 2ml vial of fludarabine is readily manufactured by Areva Pharmaceuticals in the country, which is suitable for treating cancer. In this regard, the October 2023 ASPE report indicated that its previous WAC pricing package was USD 110.0, and the latest is USD 2,736. Therefore, the changing amount accounts for USD 2,26, further denoting a 2,387.2% change rate. Thereby, with the presence of organizations producing sodium-specific drugs caters to a positive impact on the market in the country.
The rabeprazole sodium market in Canada is also growing, which is propelled by the government’s contribution to the overall healthcare well-being. According to the July 2025 Government of Canada report, there has been the provision of USD 173 million in Medicago to augment the virus-based particle vaccine and develop a large-scale biomanufacturing unit. In addition to this, the USD 27 million provision, as of March 2022, was offered to effectively support the latest capabilities and initiate progression in manufacturing facility construction within the country. Therefore, with such generous provision, the market is projected to gain exposure in the country.
Sodium-Based Drugs Pricing Comparison in North America
|
U.S. |
Canada |
||
|
Sodium Drug Type |
Price (USD) |
Sodium Drug Type |
Price (USD) |
|
Ceftriaxone sodium |
0.8 (ESI) 0.6 (Medicare) |
Jam Rabeprazole |
0.06 (LCA) |
|
Normal saline solution |
15.6 (ESI) 2.7 (Medicare) |
Pariet |
0.06 (LCA) |
|
Ondansetron |
2.2 (ESI) 0.1 (Medicare) |
Sandoz |
0.1 (LCA) |
|
Dexamethasone |
0.5 (ESI) 0.1 (Medicare) |
Oral-centric tablet |
0.1 (LCA) |
Sources: NLM, February 2023; Government of Alberta, 2025
APAC Market Insights
Asia Pacific in the rabeprazole sodium market is expected to emerge as the fastest-growing region, wth a share of 25.1%, attributed to government-based generic drug policies, extension in over-the-counter sales, a boom in localized API manufacturing, and digital health integration. According to an article published by NLM in May 2023, the overall OTC market in India was USD 3.9 billion in 2021, denoting a 6.6% increase since 2020, which is positively impacting the market in the region, based on the availability of different OTC-specific products in the healthcare system. Besides, the March 2024 NLM report stated that internationally, 60.5% of API production is in the Asia Far East, which is yet another driver for the market’s growth.
The rabeprazole sodium market in China is increasingly growing, owing to an increase in spending through NMPA-based policies, along with a surge in the patient pool. In this regard, the December 2022 NLM report, a clinical study was conducted on 276,014 patients residing in 24 provinces in China, and it was demonstrated that the overall GRED incidence rate was 8.7%. In addition, there has been an increase in the prevalence from 6.0% to 10.6%, and the disease is mainly common among people aged 40 to 60 years, with a body mass index of more than or equal to 24, thus suitable for the market demand in the country.
The rabeprazole sodium market in India is also growing due to a surge in generic drug dominance, government healthcare reforms, and a rise in rural healthcare services. As per the June 2024 PIB Government report, an estimated 96% of urban and 95% of rural population is aware of AYUSH, while 53% of urban and 46% of rural patients have utilized AYUSH for overcoming ailments within a year. Meanwhile, the January 2023 Rural India Organization report, there has been development of 31,053 primary health facilities across rural and urban locations, as of March 2022, and almost 24,935 PHCs were spotted in rural and 6,118 in urban areas, thereby enhancing the market’s exposure in the overall country.
Market Share of Different OTC Products in Asia Pacific
|
OTC Products |
% |
|
Vitamins, Minerals, and Supplements |
33.8 |
|
Gastrointestinal |
20.9 |
|
Cough, Cold, and Allergy |
14.6 |
|
Analgesics |
13.7 |
|
Dermatological |
13.4 |
|
Lifestyle CHC |
3.5 |
Source: NLM, May 2023
Europe Market Insights
Europe in the rabeprazole sodium market is projected to hold a considerable share of 28.2% during the forecast duration. The market’s development in the region is propelled by cross-border generic reforms, regional green pharma strategies, and diagnostics advancements. As per an article published by the Open Access Government Organization in September 2022, an increase in the utilization of in vitro diagnostic (IVD) has positively influenced more than 70% of medical decisions and simultaneously accounts for 0.8% of healthcare spending. In addition, the aspect of innovation in health diagnostics has transitioned testing services from hospitals to patients, and 57% of clinicians, along with 77% of domestic organizations, listed their products under this initiative.
The rabeprazole sodium market in Germany is gaining increased traction, owing to the presence of a diagnostic-based prescription model, research leadership, hospital-driven distribution, and premium price acceptance. According to the August 2025 ITA report, there has been the provision of a USD 172 billion fund, generated through international sales, of which 8.1% has been contributed to the overall healthcare system in the country, as of 2024. Additionally, in the same year, the valuation of healthcare imports was USD 188.5 billion. Therefore, with the offering of such generous fund, there is a huge opportunity for the rabeprazole sodium market to successfully flourish in the country.
The rabeprazole sodium market in the UK is also gaining increased exposure due to NHS-based affordable mandates, a surge in telemedicine, and localized supply chain facilities. As per the January 2024 NLM article, a clinical evaluation was conducted on 27 studies regarding primary, secondary, and specialized telemedicine health services that were taken into consideration. The study demonstrated that the satisfaction level ranged from 73.3% to 100% on a scoring scale of 0 to 10. Therefore, this denotes the increasing demand for telemedicine services in the country, which will make rabeprazole sodium drugs readily available for patients.
Key Rabeprazole Sodium Market Players:
- Teva Pharmaceutical (Israel)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Sun Pharmaceutical (India)
- Dr. Reddy’s Laboratories (India)
- Sandoz (Novartis) (Switzerland)
- Mylan (Viatris) (USA)
- Aurobindo Pharma (India)
- Lupin Limited (India)
- Hikma Pharmaceuticals (UK)
- Cipla (India)
- Fresenius Kabi (Germany)
- Amneal Pharmaceuticals (USA)
- Stada Arzneimittel (Germany)
- Aspen Pharmacare (South Africa)
- Krka Group (Slovenia)
- Alkaloid AD (North Macedonia)
The global rabeprazole sodium market is extremely competitive and united, with the existence of organizations, such as Dr. Reddy's and Sun Pharma, garnering the overall supply chain facility through cost-effective APIs. Besides, Sandoz and Teva represent the West market through PBM collaboration and partnerships, while Eisai and Takeda from Japan have effectively maintained a premium pricing policy for the branded version of the drug. Meanwhile, sustainability, market expansion, differentiation, and cost leadership are other strategies that these companies have implemented, thereby readily boosting the market demand and exposure internationally.
Here is a list of key players operating in the global market:
Recent Developments
- In June 2024, Sun Pharmaceutical Industries Limited notified that it has successfully entered into a patent licensing agreement with Takeda Pharmaceutical Company Limited to advertise Vonoprazan tablets 10 mg, 20 mg in India, which is suitable for aiding gastrointestinal diseases.
- In September 2023, Sanofi announced that the U.S. FDA approved the supplemental Biologics License Application (sBLA) for Dupixent, which is effective to aid children suffering with eosinophilic esophagitis (EoE).
- Report ID: 8008
- Published Date: Aug 19, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Rabeprazole Sodium Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




