Prostate Cancer Diagnostics Market Regional Analysis:
North America Market Analysis
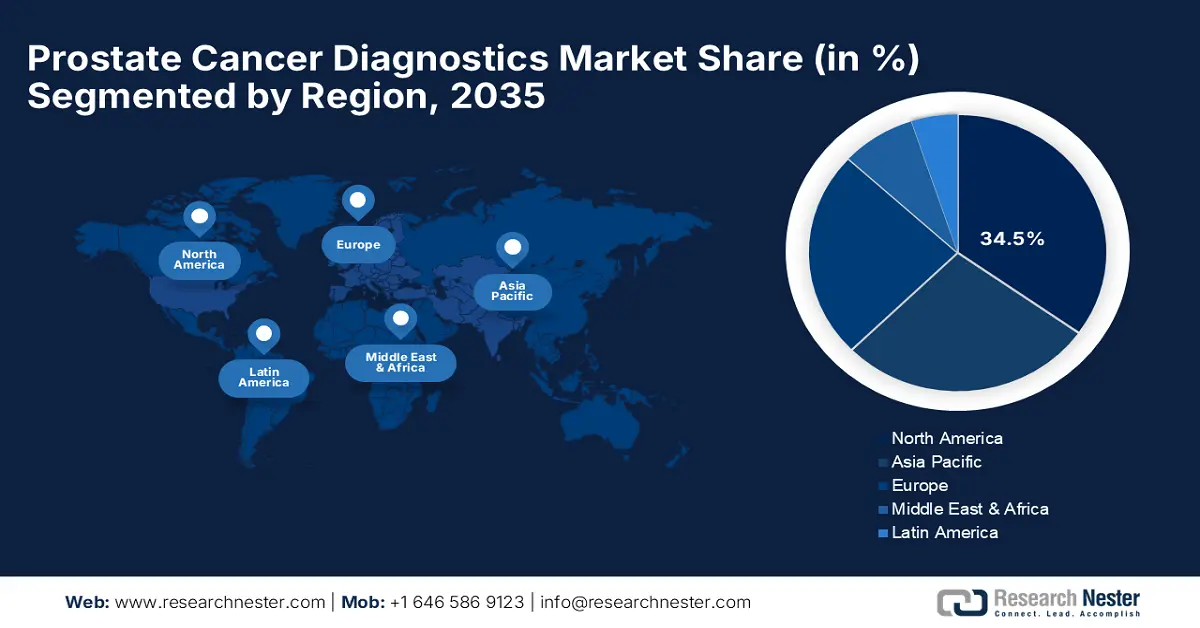
North America industry is set to hold largest revenue share of 34.5% by 2035, driven by advanced healthcare infrastructure, coupled with high incidence rates for cancer and strong support from the government. Furthermore, increased public awareness and proactive screening methods have made North America a lucrative market for companies in the prostate cancer diagnostics market. The U.S. and Canada continue to lead in North America, holding investment prospects for new players.
The U.S. prostate cancer diagnostics market demonstrates strong adoption of advanced diagnostic modalities since the country carries out large-scale screening programs and has substantial funding from the government. For example, Lantheus Holdings announced revenues of over USD 150 million for PYLARIFY, its leading PSMA PET imaging test for prostate cancer, in March 2024. Strong revenue indicates high demand for advanced diagnostic solutions in the U.S., driven by early diagnosis and precision medicine.
Canada healthcare system is becoming another leading adopter of advanced diagnostics due to governmental approvals for new technologies in cancer screening. For example, in May 2023, Health Canada approved the PSMA PET imaging agent known as Illuccix for the diagnostics of prostate cancer. Therefore, this highlights that the country is committed to promoting innovative healthcare solutions. This approval underlines that Canada is proactive in the adoption of cutting-edge diagnostic tools, hence driving North America prostate cancer diagnostics market.
Asia Pacific Market Analysis
Asia Pacific prostate cancer diagnostics market is projected to experience notable growth from 2025 to 2035. This is owing to increased investments by governments in health infrastructure and an increased focus on early detection of cancer. Furthermore, an increasing geriatric population and better access to healthcare services are opening up new avenues for diagnostics providers in the region.
India prostate cancer diagnostics market is rising at a steady pace, with the government investing more in cancer care and making greater efforts to raise public health awareness. For example, the Assam government announced in November 2023 that it would invest INR 135 crore in the Assam Cancer Care Foundation to construct ten cancer hospitals, which would increase access to diagnostics. Thus, the determination of India to counter the rising influence of cancer with better health facilities further promotes market growth.
China prostate cancer diagnostics market growth is propelled by the government’s initiatives to improve access to healthcare, along with agreements with overseas companies for the provision of diagnostics. For example, in October 2023, a collaboration between Sinotau Pharmaceutical Group and Blue Earth Diagnostics introduced a new imaging agent in China used in diagnosing prostate cancer. The collaboration represents the country's commitment to new diagnostic solutions, driving the prostate cancer diagnostics market growth through 2035.