Progressive Supranuclear Palsy Market Outlook:
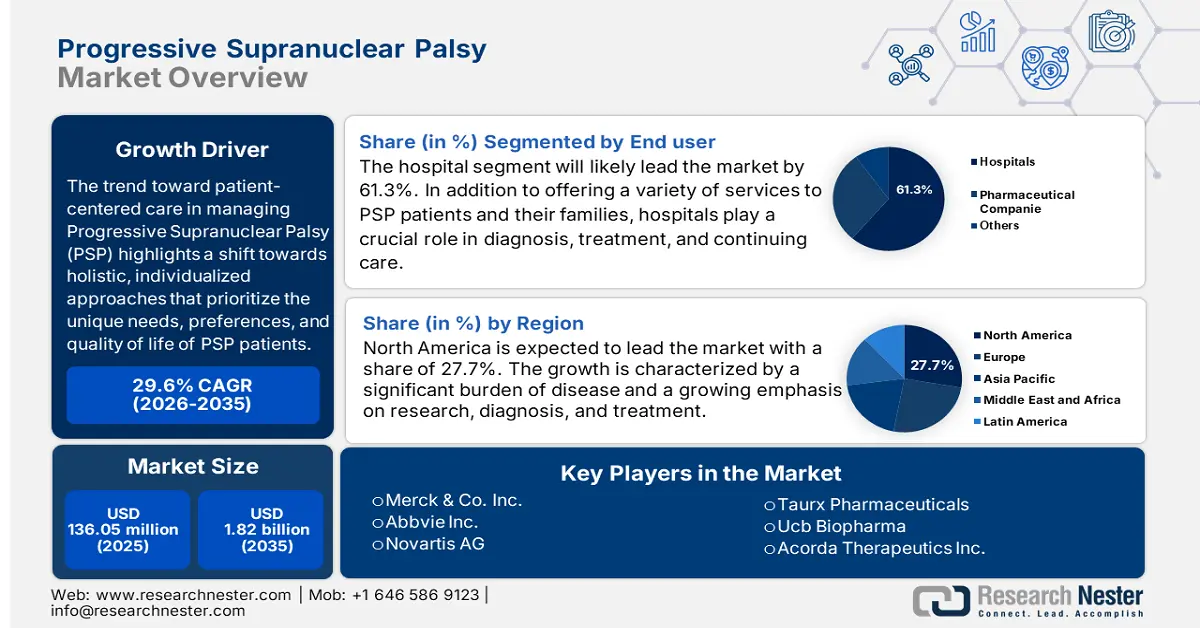
Progressive Supranuclear Palsy Market size was over USD 136.05 million in 2025 and is projected to reach USD 1.82 billion by 2035, witnessing around 29.6% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of progressive supranuclear palsy is evaluated at USD 172.29 million.
The progressive supranuclear palsy market is growing rapidly due to the increasing clinical and research knowledge of PSP which spurs the need for new treatments. Moreover, clinical trials of experimental therapies, such as tau aggregation inhibitors, are encouraging for better disease management. For instance, as per the Eurek Alert Organization October 2024 report, researchers created a medication that targets the two main hotspots of the Tau protein in the brain that promote aggregation. In both laboratory and fruit fly experiments, the medication, a peptide inhibitor known as RI-AG03, successfully stopped the accumulation of Tau proteins. Hence, patients have better access to specialized care owing to the development of telemedicine, making remote monitoring and consultations feasible.
Furthermore, the rising incidences of market are driving demand. For instance, according to studies by NLM in August 2022, there were approximately 6 cases of PSP for every 100,000 people which ranged between 0.9 and 2.6 incidences per 100,000 people each year. In addition, increasing awareness, improving early diagnosis, and supporting PSP research have all been made possible by the growth of patient advocacy and support groups. These elements reflect a promising future for PSP treatment options and highlight the necessity of continued funding for public health initiatives and research.
Key Progressive Supranuclear Palsy Market Insights Summary:
Regional Highlights:
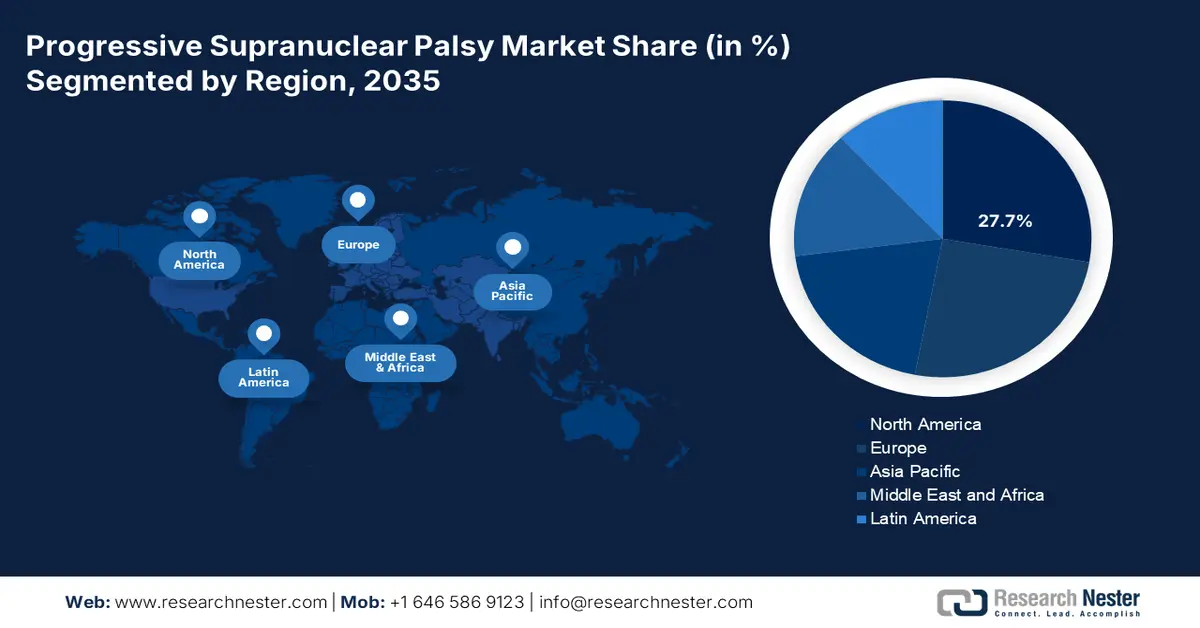
- North America leads the Progressive Supranuclear Palsy Market with a 27.7% share, supported by a developed healthcare system and significant R&D investment enabling cutting-edge therapies, driving growth through 2035.
- Asia Pacific's progressive supranuclear palsy market anticipates lucrative growth by 2035, attributed to aging populations, growing healthcare investments, and increased focus on neurological care.
Segment Insights:
- The Hospital segment of the Progressive Supranuclear Palsy Market is expected to hold a 61.3% share by 2035, fueled by its ability to provide specialized care for neurological disorders.
- The treatment segment of the Progressive Supranuclear Palsy Market is expected to be the most crucial area of growth from 2026-2035, fueled by the growing emphasis on personalized medicine tailored to patient needs.
Key Growth Trends:
- Increased funding for PSP research
- Telemedicine and remote monitoring
Major Challenges:
- Clinical trial difficulties
- Patient care coordination
- Key Players: Novartis AG, Pfizer Inc., Alpine Life Sciences Private Limited, Varian Medical Systems, Inc., Siemens Healthineers AG, Acorda Therapeutics Inc., and more.
Global Progressive Supranuclear Palsy Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 136.05 million
- 2026 Market Size: USD 172.29 million
- Projected Market Size: USD 1.82 billion by 2035
- Growth Forecasts: 29.6% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (27.7% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, Germany, Japan, United Kingdom, France
- Emerging Countries: China, Japan, India, South Korea, Brazil
Last updated on : 13 August, 2025
Progressive Supranuclear Palsy Market Growth Drivers and Challenges:
Growth Drivers
-
Increased funding for PSP research: An important factor propelling the progressive supranuclear palsy market expansion is enhanced funds for research, which holds lucrative opportunities for innovative treatment developments and improved understanding of the condition. For instance, in November 2024, INBRAIN Neuroelectronics, which creates neural technologies based on graphene, announced that a USD 50 million Series B funding round has closed. In addition to expediting clinical trials, this increased funding promotes cooperation among researchers, physicians, and advocacy organizations. Therefore, the funding infusion plays a crucial role in expanding the role of PSP, leading to better management choices and better patient outcomes.
- Telemedicine and remote monitoring: The progressive supranuclear palsy market is expanding in large part due to telemedicine and remote monitoring, which ensure patients with access to specialized care. Healthcare providers are able to reach patients in underserved and rural areas by offering virtual consultations, thus removing geographic barriers to high-quality care. For instance, in April 2022, a nationwide study by the National Library of Medicine revealed that private insurance claims data from 36 million working-age people, and telemedicine encounters rose by 766% during the first three months of the pandemic. This is 0.3% of all interactions between March and June 2019 to 23.6% of all interactions during that time.
Challenges
-
Clinical trial difficulties: The progressive supranuclear palsy market is plagued by clinical trials difficulty, primarily due to the disease's rarity. Typically, the low number of diagnosed patients hinders recruitment for clinical trials, leading to long study enrollment periods and ultimately delaying the development of new treatments. In addition, the variability of PSP symptoms makes it challenging to standardize trial protocols, which impedes reliable data collection and consistent participation. These difficulties underscore the need for creative trial design and multidisciplinary cooperation to maximize recruitment and retention tactics and accelerate the progress of PSP clinical research.
- Patient care coordination: Neurologists, primary care doctors, physiotherapists, and other specialists fail to treat progressive supranuclear palsy (PSP) with a multidisciplinary approach, making patient care coordination a crucial challenge in the market. Due to the need for efficient coordination and communication between various specialists, care is frequently fragmented, leading to inconsistent treatment plans and patient experiences. Furthermore, caregivers may experience challenges navigating the healthcare system, leading to gaps in resources and support for individuals with PSP.
Progressive Supranuclear Palsy Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
29.6% |
|
Base Year Market Size (2025) |
USD 136.05 million |
|
Forecast Year Market Size (2035) |
USD 1.82 billion |
|
Regional Scope |
|
Progressive Supranuclear Palsy Market Segmentation:
End User (Hospitals, Pharmaceutical Companies)
Hospital segment is likely to capture progressive supranuclear palsy market share of over 61.3% by 2035, timeline due to its ability to provide care to patients with neurological disorders. For instance, in July 2023, the Rohini Nilekani Centre for Brain and Mind (CBM) was inaugurated by the National Institute of Mental Health and Neurosciences (NIMHANS) and the National Centre for Biological Sciences (NCBS) in Bengaluru. The institute was given a milestone-based grant of USD 1.5 billion through the philanthropic organization. In addition to facilitating timely intervention and management, this convergence of resources and expertise highlighted the critical role that hospital-based treatment plays.
Application (Treatment)
Based on application, the treatment segment is one of the most crucial areas of growth in the progressive supranuclear palsy market. It includes all therapeutic approaches for managing the degenerative condition's symptoms and progression. The importance of effective symptom management and quality of life enhancement for PSP patients is reflected in the treatment focus. For instance, in May 2024, Transposon Therapeutics announced that the U.S. FDA granted it fast-track designation for an intervention designed to treat PSP. This segment's dominance is further reinforced by the growing emphasis on personalized medicine, which allows physicians to tailor treatment plans to each patient's unique needs and responses.
Our in-depth analysis of the global progressive supranuclear palsy market includes the following segments:
|
Treatment Modality |
|
|
Application |
|
|
End User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Progressive Supranuclear Palsy Market Regional Analysis:
North America Market Statistics
North America progressive supranuclear palsy market is estimated to capture revenue share of over 27.7% by 2035. The developed healthcare system and significant investment in research and development create a favorable environment for the development of cutting-edge therapies. Therefore, North America's proactive approach to addressing the difficulties associated with this complex neurodegenerative illness is what defines its market dominance in PSP.
The U.S. progressive supranuclear palsy market’s profitable growth is due to its robust pharmaceutical industry and extensive R&D capacity. For instance, in October 2024, the U.S. FDA approved VYALEV (foscarbidopa and foslevodopa) produced by AbbVie as the first and only subcutaneous 24-hour infusion of levodopa-based therapy. This treats motor fluctuations in adults with advanced Parkinson's disease (PD). To guarantee that patients receive state-of-the-art therapies and clinical treatments, this emphasis on development supports treatment advancements.
The progressive supranuclear palsy market in Canada is witnessing significant growth due to strategic investments and partnerships to foster advancements and effective therapies. For instance, in September 2024, the government of Canada and its partners, Heart & Stroke and Brain Canada announced to invest USD 10 million to create two new national research networks for women's heart and brain health. It aimed at establishing a national partnership to lower the number of fatalities and severe illnesses caused by heart conditions during and after pregnancy.
Asia Pacific Market Analysis
The progressive supranuclear palsy market in Asia Pacific is gaining traction and is expected to witness lucrative growth during the forecast timeline i.e. 2026-2035. A greater focus is being placed on diagnosis and treatment in the region because PSP and other neurological disorders are expected to become more common as the population ages. Additionally, a thriving environment for advancements in clinical practice and therapeutic innovation has been established by growing investments in the healthcare infrastructure and research initiatives.
The progressive supranuclear palsy market in India is expanding due to its regional high healthcare infrastructure investment and rising awareness of neurodegenerative diseases. For instance, in January 2025, according to a Morgan Stanley report unveiled by the India Brand Equity Foundation, India's infrastructure investment is expected to rise gradually from 5.3% of GDP in FY24 to 6.5% of GDP by FY29. Furthermore, more access to professional neurological treatment and advanced diagnostic facilities for PSP has been made possible by the expanding healthcare sector, which has been bolstered by both public and private investments.
The progressive supranuclear palsy market in China is gaining noteworthy traction owing to China's rapidly aging population and rising healthcare spending. For instance, as per an analytical study conducted by NLM in September 2024, an estimated 61.41% of China's total health expenditures (institutional method), were allocated to hospital expenses in 2022. Hence, the incidence of neurodegenerative diseases such as PSP will rise due to the massive aging of the population, which will push the government to prioritize neurological care and emphasize building healthcare infrastructure and raising public awareness of PSP.
Key Progressive Supranuclear Palsy Market Players:
- Merck & Co., Inc.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Novartis AG
- Pfizer Inc.
- Alpine Life Sciences Private Limited
- Varian Medical Systems, Inc.
- Siemens Healthineers AG
- Acorda Therapeutics Inc.
- Asceneuron Sa
- Taurx Pharmaceuticals
- Sun Pharmaceutical Industries Limited
- GSK plc
The progressive supranuclear palsy market is characterized by numerous significant players that speed up and improve the efficacy of clinical trials, facilitating the introduction of novel therapies and advancing our understanding of PSP in general. For instance, in September 2024, Ferrer, an international pharmaceutical company with B Corp certification, revealed the dosage for PROSPER, the first participant in the Phase II clinical trial. The purpose of the study was to assess the safety and effectiveness of FNP-223,1 a novel therapy designed to slow the progression of PSP.
Here's the list of some key players:
Recent Developments
- In November 2024, Axonis Therapeutics rewired a virus to deliver a new gene therapy to neurons to treat neurological disorders such as spinal cord injury, Parkinson's disease, and Alzheimer's.
- in May 2024, BrainTale expanded its footprints globally and revealed tier-one partnerships to enhance brain health and facilitated precision neurology.
- Report ID: 7210
- Published Date: Aug 13, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




