Polymerase Chain Reaction Market Outlook:
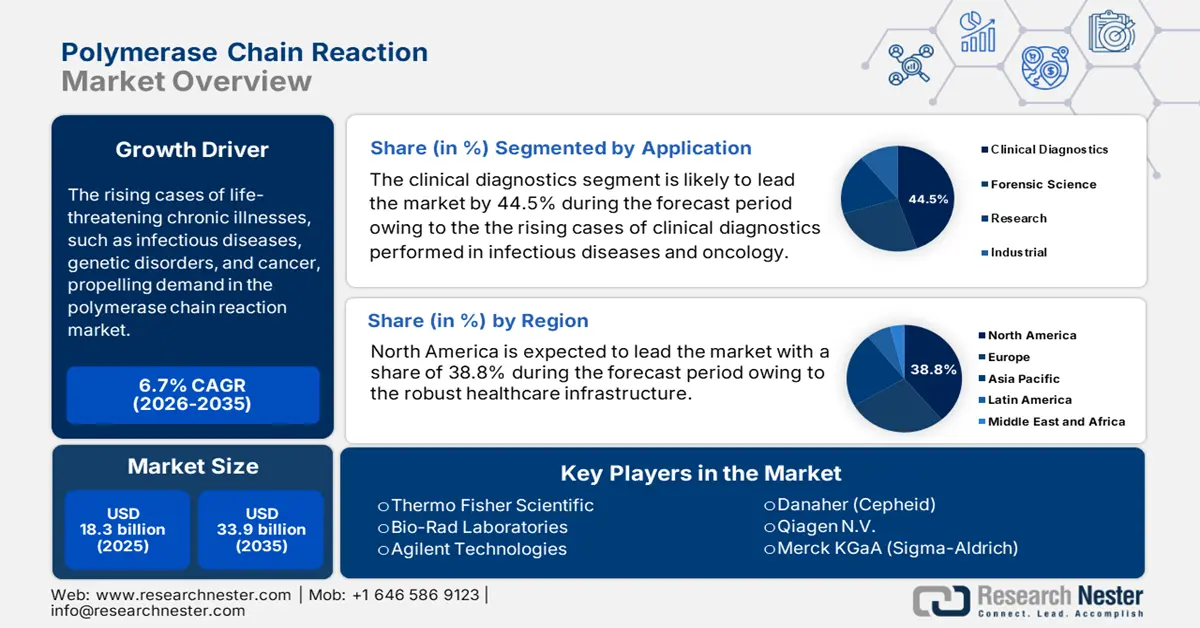
Polymerase Chain Reaction Market size was valued at USD 18.3 billion in 2025 and is projected to reach USD 33.9 billion by the end of 2035, rising at a CAGR of 6.7% during the forecast period, i.e., 2026-2035. In 2026, the industry size of polymerase chain reaction is evaluated at USD 19.4 billion.
The polymerase chain reaction market is driven by the patient pool in infectious disease cases, oncology, and public health surveillance. The NIH publication issued in November 2021 indicates that close to 1.1 million PCR tests have been conducted for COVID-19 and points to the necessity for PCR tests during the pandemic. Nearly 10.6 million people were affected by TB in 2022, where nucleic acid amplification tests are vital for screening and detection, as per the 2025 WHO report. FDA’s IVD policies surges the quality, validation, and postmarket controls for PCR platforms used by clinical labs and manufacturers.
Supply chain resilience for PCR products embraces biologicals (polymerases, RTs), oligos/primers/probes, plastics (tips, tubes, plates), electronics/optics, and assembled instruments. FDA device listing and registration data indicate a wide base of U.S. and foreign facilities for IVD reagents and analyzers, and UN Comtrade trade data indicate extensive cross-border flows in HS 3822 (diagnostic/lab reagents) and HS 9027 (instruments). Further, the OEC report in 2023 highlights, the U.S. is the leader in exporting lab reagents and exported worth USD 108 million in 2023. Specialty enzyme and oligonucleotide import/export dependencies remain; assembly is normally conducted within certified facilities under ISO 13485 and FDA QSR/21 CFR Part 820.