Pneumonia Testing Market - Regional Analysis
North America Market Insights
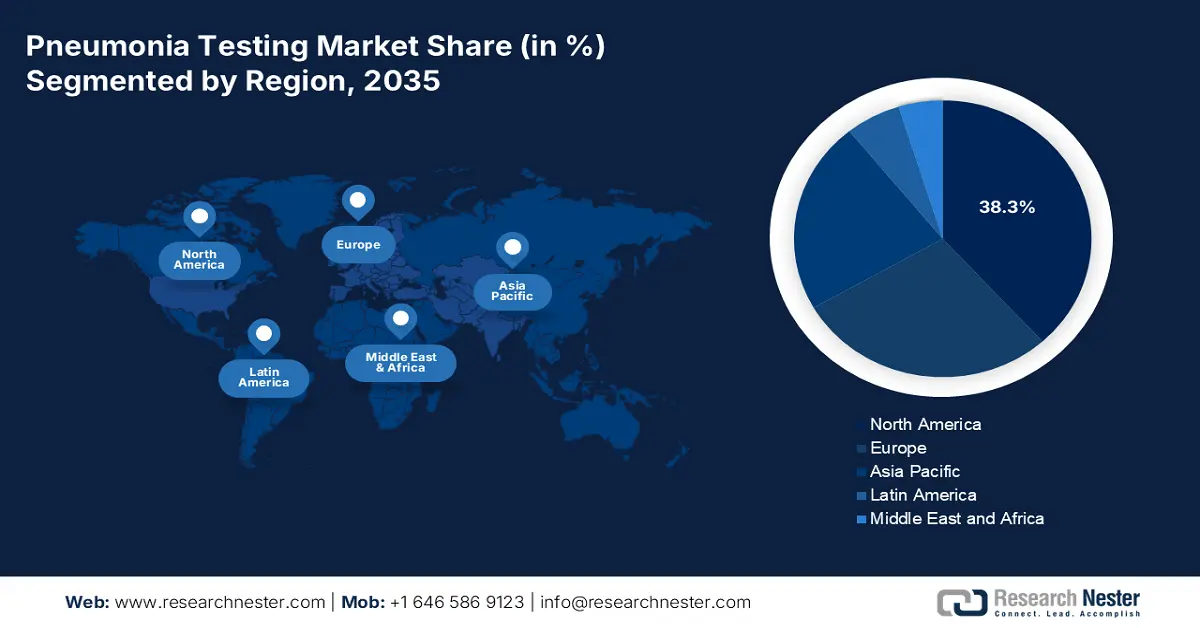
North America in the pneumonia testing market is projected to be the dominant region, grabbing the largest share of 38.3% by the end of 2035. The market’s growth in the region is effectively fueled by innovative diagnostic integration, the presence of federal funding, and a surge in the incidence rate. According to the December 2024 ASPE Organization report, Illumina, within the past 7 years, unveiled the iSeq Sequencing system, which is a benchtop sequencer, for an estimated USD 20,000. In addition to this, the organization also introduced high-throughput sequencers, with a valuation of USD 1 million, thereby focusing on a diagnostic approach, which is suitable for the market’s growth in the overall region.
The pneumonia testing market in the U.S. is significantly growing, owing to the expansion in POC testing, artificial intelligence-based diagnostic tools, and Medicare and Medicaid reimbursement policies. As per an article published by Frontiers Organization in May 2024, the overall North America region represented itself as the top frontrunner in the POC testing industry, accounting for a market share of 43.2% as of 2022, which is readily driving the pneumonia testing market in the country. Besides, as per the June 2025 CDC Government report, the Health Care and Education Reconciliation Act (HCERA), along with the Patient Protection and Affordable Care Act (ACA) made effective modifications in the Medicaid reimbursement policies, which is also positively impacting the market in the country.
The pneumonia testing market in Canada is also growing due to federal provisions, as well as an increase in Ontario’s expenditure. Besides, the February 2022 NLM article has put forward the antimicrobial activity for bacterial CAP pathogens, including 98% to 100% of S pneumoniae strains, 94% for cefuroxime, 85% for doxycycline, and 78% for macrolide, all of which develop outside hospital settings. However, Ontario has provided generous investment for hospital facilities, suitable for overcoming the pneumonia incidence. According to the April 2025 Ontario government report, the government has supported more than 50 hospitals and 3,000 beds in the past 10 years. Additionally, there has been an investment of USD 50 billion for the coming 10 years for hospital infrastructure, which is suitable for the overall market.
Recommended Plans for Pneu-C-15 or Pneu-C-20 Vaccine for Children in Both the U.S. and Canada
|
Age at presentation for immunization |
Number of pneumococcal conjugate vaccine doses previously received |
Suggested schedule for Pneu-C-15 or Pneu-C-20 |
|
2 to less than 7 months |
0 doses |
2 or 3 doses + 1 dose at 12 to 15 months of age |
|
1 dose |
1 or 2 doses + 1 dose at 12 to 15 months of age |
|
|
2 doses |
0 or 1 dose + 1 dose at 12 to 15 months of age |
|
|
7 to less than 12 months |
0 doses |
2 doses + 1 dose at 12 to 15 months of age |
|
1 dose |
1 dose + 1 dose at 12 to 15 months of age |
|
|
2 doses |
1 dose at 12 to 15 months of age |
|
|
12 to less than 24 months |
0 doses |
2 doses |
|
1 dose at less than 12 months of age |
||
|
2 or more doses at less than 12 months of age |
1 dose |
|
|
0 or 1 dose at less than 12 months of age AND 1 dose at 12 months of age or older |
||
|
24 to less than 60 months (5 years) |
0 doses or incomplete vaccination schedule |
1 dose |
|
5 to less than 18 years |
0 doses |
0 doses |
Source: Government of Canada, May 2024
APAC Market Insights
Asia Pacific in the pneumonia testing market is anticipated to be the fastest-growing region, garnering a share of 22.5% during the forecast timeline. The market’s upliftment in the region is highly driven by a surge in the disease burden, governmental strategies, and private sector funding. According to the November 2024 Observer Research Foundation Organization report, the death toll for pneumonia in India was over 127,000 children under 5 years of age, accounting for 14% of deaths in this particular age group. However, to combat this, treatment expenses constitute USD 25.6 per episode, especially for elderly patients. Therefore, all these factors are responsible for uplifting the market in the overall region.
The pneumonia testing market in China is gaining increased exposure, owing to an increase in the aging population, governmental funding, and technology-based diagnostic solutions. As per an article published by NLM in October 2024, the digital healthcare market in the country has reached CNY 195.4 billion, as of 2022, with a growth rate of 30% over the past 5 years. In addition, there has been the establishment of 125 regional medical facilities, particularly in Tier-2 and Tier-3 cities. Furthermore, the electronic medical record (EMR) coverage accounted for 90% of hospitals, followed by 60% secondary hospitals, and 40% primary hospitals, thus denoting a huge opportunity for the market’s development.
The pneumonia testing market in India is steadily growing since there has been an expansion in governmental initiatives, development in telemedicine services, and a boost in local manufacturing. As per the December 2024 PIB Government report, Nafithromycin has been specifically designed, with a fund provision of ₹8 Crore (USD 913,371), to aid community-acquired bacterial pneumonia (CABP). This has been possible by conducting Phase III clinical trials under the Biotechnology Industry Research Assistance Council (BIRAC) Biotech Industry Program supervision, which is a huge growth opportunity for the market in the country.
Total Deaths and Years of Full Health (YFH) in Asia
|
Pathogens |
Total deaths averted (till 2024) |
Total YFH gained (till 2024) |
||
|
|
All Ages |
Under 5 years |
All Ages |
Under 5 years |
|
Diphtheria |
81,000 |
73,000 |
5,430,000 |
4,900,000 |
|
Haemophilus influenzae type B |
601,000 |
601,000 |
43,365,000 |
43,365,000 |
|
Hepatitis B |
73,000 |
57,000 |
10,486,000 |
8,158,000 |
|
Measles |
20,517,000 |
20,194,000 |
1,264,122,000 |
1,244,255,000 |
|
Pertussis |
4,428,000 |
3,910,000 |
366,568,000 |
323,138,000 |
|
Poliomyelitis |
347,000 |
196,000 |
168,577,000 |
95,333,000 |
Source: NLM, November 2024
Europe Market Insights
Europe in the pneumonia testing market is projected to account for a considerable share of 28.4% by the end of the forecast period. The market’s upliftment in the region is propelled by the presence of health policies, effective import and export facilities, POCT integration, and stringent monitoring facilities. According to the 2023 OEC data report, France is one of the top exporters of oxygen, with a valuation of USD 26.5 million. This is followed by Belgium, accounting for USD 22.1 million, USD 17.9 million in the Netherlands, and USD 14.3 million in Slovakia. Besides, primary as well as hospital care are provided to patients with no cost, which is an optimistic outlook for the overall market in the region.
The pneumonia testing market in Germany is steadily growing, owing to an increase in healthcare expenditure, automation in hospitals, continuous research and development activities, and Europe Union funding. As indicated in the 2025 World Bank Group data report, the country’s gross domestic product (GDP) rate for healthcare spending is 11.8% as of the financial year 2022. Besides, the 2023 OECD Organization report stated that the health expenditure in the country amounted to EUR 159 per capita, along with an upsurge in public funding by 85.5% for the healthcare system, denoting an increase from 81.1%. Additionally, the out-of-pocket aspect is lower by 12% in the country, thereby effectively bolstering the market.
The pneumonia testing market in the UK is also propelling due to the presence of NHS investment, an expansion in telemedicine, localized production, and public-private collaborations. As per an article published by Informatics and Health in March 2025, the NHS reported that there has been an increase in telemedicine usability in the country by 67%, particularly in rural locations. For instance, Cumbria witnessed a surge by 45% since pandemic days, which has permitted patients to achieve consultations within the required duration. Therefore, the availability of this facility has developed a positive outlook for the overall market in the country.