Phenylephrine Hydrochloride Market Outlook:
Phenylephrine Hydrochloride Market size was over USD 962.51 million in 2025 and is anticipated to cross USD 2.06 billion by 2035, witnessing more than 7.9% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of phenylephrine hydrochloride is assessed at USD 1.03 billion.
The phenylephrine hydrochloride market is expanding due to the growing prevalence of seasonal allergies such as nasal congestion, sinusitis, common cold, and allergies. According to the National Institutes of Health (NIIH), one of the most prevalent chronic illnesses in the world, chronic rhinosinusitis (CRS) affects people of all ages. The estimated incidence rates in the USA, Europe, and China are 12.3%, 10.9%, and 13%, respectively. A basic component of the pharmaceutical business, phenylephrine HCL is an essential constituent in many prescription and over-the-counter drugs used to treat nasal congestion. Additionally, the phenylephrine hydrochloride market gains from phenylephrine HCL's versatility in various forms, such as liquids, oral pills, and nasal sprays.
Key Phenylephrine Hydrochloride Market Insights Summary:
Regional Highlights:
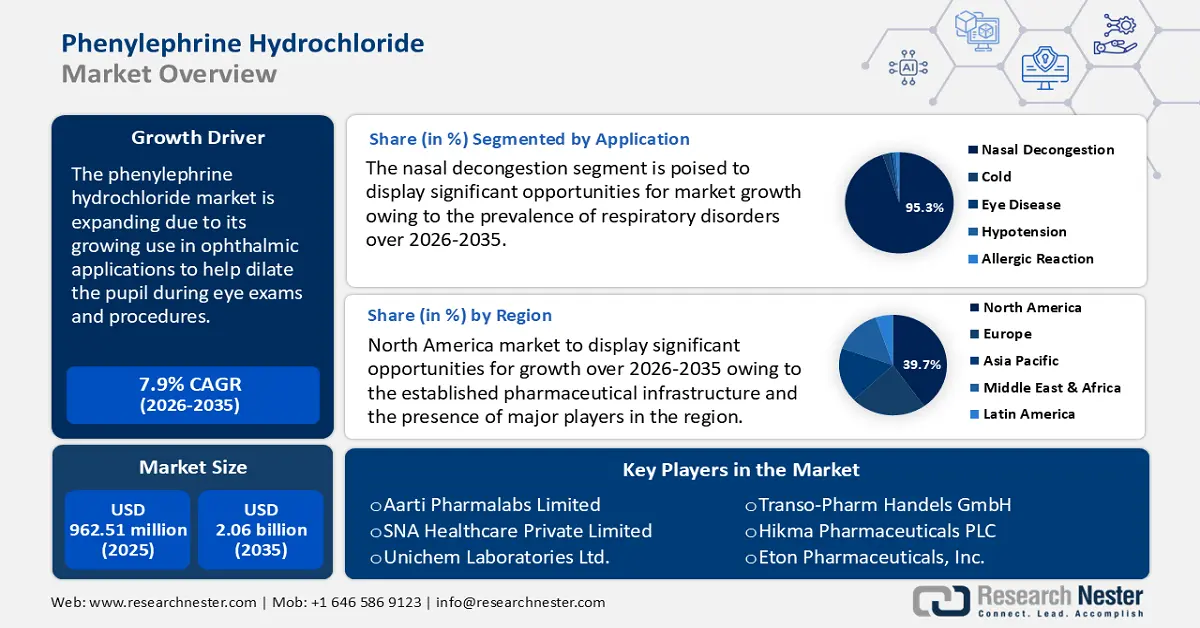
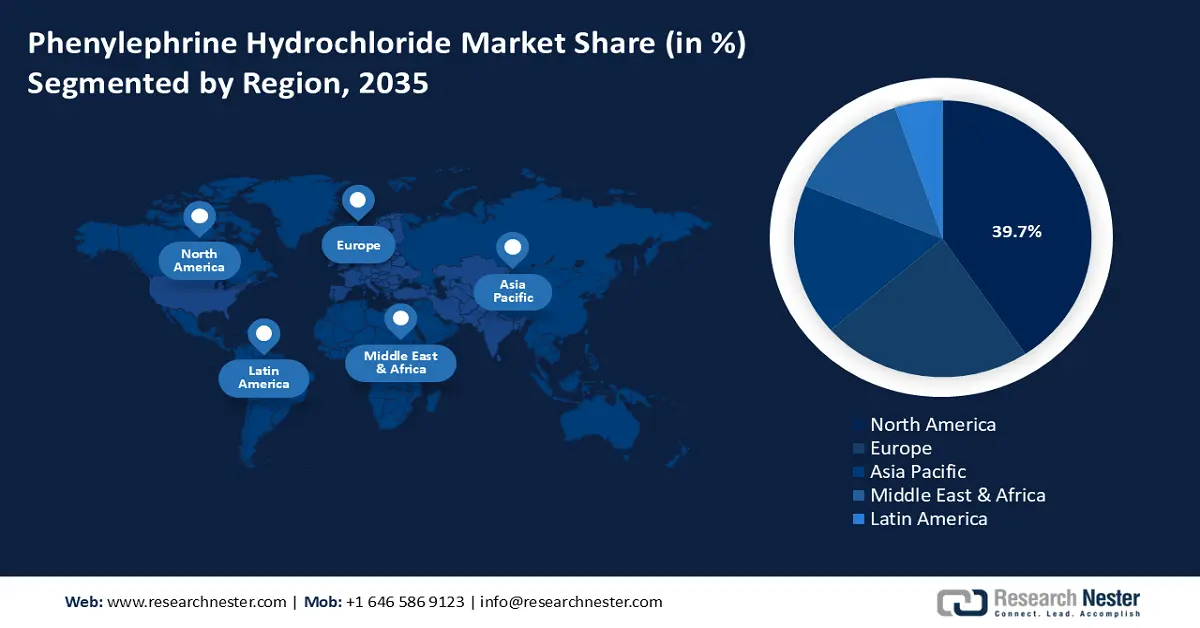
- North America in the phenylephrine hydrochloride market is predicted to hold a 39.7% revenue share by 2035, driven by the growing adoption of online pharmacies and self-medication trends.
- Europe is expected to witness substantial growth by 2035, owing to the rising prevalence of allergy-related disorders exacerbated by climate change.
Segment Insights:
- Nasal decongestion segment in the phenylephrine hydrochloride market is projected to account for 95.3% share by 2035, propelled by seasonal reasons and the year-round prevalence of ailments including sinus congestion, allergies, and the common cold.
- In-house manufacturing segment is expected to gain significant traction by 2035, owing to streamlined integration between R&D and production processes.
Key Growth Trends:
- Increasing use in ophthalmic applications
- Growing use in the treatment of priapism
Major Challenges:
- Adverse effects of the medication
- Regulatory obstacles
Key Players: Aarti Pharmalabs Limited, SNA Healthcare Private Limited, Shenzhen Oriental Pharmaceutical Co., Ltd., Unichem Laboratories Ltd., Transo-Pharm Handels GmbH, Hikma Pharmaceuticals PLC, Eton Pharmaceuticals, Inc., LGM Pharma, AstraZeneca plc, Abbott Laboratories.
Global Phenylephrine Hydrochloride Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 962.51 million
- 2026 Market Size: USD 1.03 billion
- Projected Market Size: USD 2.06 billion by 2035
- Growth Forecasts: 7.9%
Key Regional Dynamics:
- Largest Region: North America (39.7% Share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, Germany, France, Japan, Canada
- Emerging Countries: China, India, Brazil, Mexico, South Korea
Last updated on : 2 December, 2025
Phenylephrine Hydrochloride Market - Growth Drivers and Challenges
Growth Drivers
- Increasing use in ophthalmic applications: Phenylephrine HCL is a well-known application in nasal decongestant drugs, it is becoming relevant in several medical and non-medical fields. Phenylephrine HCL has found use in ocular applications in addition to respiratory health. It is used as a mydriatic agent in ophthalmic solutions to help dilate the pupil during eye exams and procedures. This feature makes the inside parts of the eye more visible, which helps surgeons and other medical experts with diagnosis and surgery. For pupil dilation, the U.S. Food and Drug Administration (FDA) has authorized the use of up to three drops of phenylephrine hydrochloride ophthalmic solution at 2.5% or 10%.
- Growing use in the treatment of priapism: Usually ischemic, sickle cell disease (SCD) is the primary cause of priapism in children and teenagers. According to reports, 18–27% of boys with SCD experience priapism. Twelve years is the usual age for presentation, and each patient experiences 15 episodes on average over their lives. A prolonged penile erection that lasts for hours after or without sexual stimulation is known as priapism. The American Urological Association (AUA) and Sexual Medicine Society of North America (SMSNA) recommend phenylephrine, a pure alpha-adrenergic agonist, as the preferred sympathomimetic drug for treating ischemic priapism because of its quick onset and brief duration of action. Because of its alpha-1 selectivity, phenylephrine's cardiovascular adverse effects are less likely to occur than those of other sympathomimetic drugs.
- Advances in drug delivery systems: Advanced drug delivery systems are logically designed to improve the performance and distribution of current medications compared to conventional systems. Unlike traditional drug delivery systems, novel drug delivery systems target, control, and adjust drug administration by combining innovative dosage forms and sophisticated methodologies. Researchers are examining the use of nanoparticles, targeted delivery systems, and controlled release formulations to deliver phenylephrine HCL. Moreover, transdermal delivery systems, such as patches and gels, have been developed to provide controlled drug release over an extended period. These innovations have improved the safety and efficiency of phenylephrine HCL for various conditions including nasal congestion, hypertension, and priapism.
Challenges
- Adverse effects of the medication: Skin rash, itching, hives, and swelling of the face, lips, tongue, or throat are examples of adverse reactions that the patient taking phenylephrine hydrochloride may have. In a patient who is awake, the most frequent adverse responses are headache, nausea, vomiting, and anxiety. Phenylephrine can induce baroreceptor-mediated reflex bradycardia due to its exclusive activation of alpha-receptors. When treating a patient with bradycardia and hypotension, clinicians should consider various kinds of vasopressor drugs. Additionally, it has an impact on patients' renal efficacy and heart palpitations in the elderly population. Therefore, the side effects of this formulation may impede the phenylephrine hydrochloride market.
- Regulatory obstacles: Strict regulations about the efficacy, safety, and quality of active pharmaceutical ingredients manufacture are regularly enforced by the FDA and other regulatory bodies. The FDA announced in September 2023 that nasal congestion cannot be treated with oral phenylephrine (Sudafed PE). Although they were left out of this announcement, phenylephrine nasal sprays, often known as Neo-Synephrine, are still a viable alternative. This led to an increased demand for alternative medications such as fluticasone propionate (Flonase), oxymetazoline (Afrin), and pseudoephedrine (Sudafed). Therefore, these regulations may hinder the adoption and use of phenylephrine hydrochloride in the forecast period.
Phenylephrine Hydrochloride Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
7.9% |
|
Base Year Market Size (2025) |
USD 962.51 million |
|
Forecast Year Market Size (2035) |
USD 2.06 billion |
|
Regional Scope |
|
Phenylephrine Hydrochloride Market Segmentation:
Application Segment Analysis
Nasal decongestion segment is set to capture phenylephrine hydrochloride market share of around 95.3% by the end of 2035. The segment is expanding due to seasonal reasons and the year-round prevalence of ailments including sinus congestion, allergies, and the common cold. Since it is the active ingredient that relieves nasal congestion, phenylephrine hydrochloride, or API, is an essential part of nasal decongestant drugs. By narrowing the blood vessels in the nasal passages, this medication helps to lessen the swelling and congestion brought on by several respiratory disorders, including hay fever, sinusitis, allergies, and the common cold. Additionally, a variety of nasal decongestant medications, such as nasal sprays, nasal drops, and oral tablets, include phenylephrine hydrochloride. These formulations are widely accessible over-the-counter (OTC) and by prescription, are designed to help those looking for rapid and efficient relief from nasal congestion.
Mode Segment Analysis
The in-house manufacturing segment in phenylephrine hydrochloride market will garner a notable share in the forecast period. Pharmaceutical businesses precisely manage every step of the production process when they manufacture phenylephrine hydrochloride PAI in-house. It gives pharmaceutical companies more freedom to tailor their production to meet their unique needs and market expectations. Additionally, in-house API manufacturing can facilitate seamless integration between R&D and production departments for businesses engaged in pharmaceutical research and development (R&D). By reducing lead times and streamlining the development process, this integration makes it easier to move from medication discovery to commercial production.
Our in-depth analysis of the phenylephrine hydrochloride market includes the following segments:
|
Application |
|
|
Mode |
|
|
Use |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Phenylephrine Hydrochloride Market - Regional Analysis
North American Market Insights
North America in phenylephrine hydrochloride market is anticipated to dominate over 39.7% revenue share by 2035. Phenylephrine hydrochloride API (HCl) is crucial for the production of nasal decongestant medications and plays a significant role in the pharmaceutical industry in North America. A diverse array of pharmaceutical companies and the growing adoption of online pharmacies are growing the market for phenylephrine HCl in this region. Moreover, the increasing trend of self-medication and availability of OTC medications is consequently driving market growth.
The phenylephrine hydrochloride market is expanding in the U.S. due to the increasing prevalence of sinus and seasonal allergies, and growing awareness related to the condition. According to the American College of Allergy Asthma and Immunology, Sinusitis, another name for sinus infection, is a serious medical condition. In the U.S., 31 million people suffer from it. Over USD 1 billion is spent annually by Americans on over-the-counter drugs to cure it. USD 150 million in prescription drug costs and 16 million doctor visits are attributable to sinus infections.
In Canada, due to the obesity pandemic and aging population demographics, the prevalence of hypertension will continue to climb. Obesity is similarly unmalleable on a population-wide level, and aging is unquestionably irreversible. The government assists children, youth, and families in adopting and sustaining healthy behaviors, such as regular exercise and a balanced diet, in collaboration with the provinces, territories, and partners. For instance, to enhance general health and well-being, encourage healthy living, and aid in preventing chronic diseases, the Public Health Agency of Canada supports community-based initiatives, such as those that assist young families and their children, through the Healthy Canadians and Community Fund. Therefore, the growing prevalence of hypertension is one of the major factors escalating the phenylephrine hydrochloride market in the nation.
Europe Market Insights
Europe phenylephrine hydrochloride market will encounter huge growth during the forecast period. It is anticipated that climate change will have an impact on allergy disorders, and clinical specialists believe that these diseases will rise as a result of climate change, partly due to the impact on plant species that cause allergies. The rising incidence of allergic asthma has already been attributed in part to climate change. Since the effects of climate change are complicated, there is no quantitative estimate of how future climate change may impact human pollen allergy levels, although pollens are a key cause of symptoms in those with allergic diseases. Therefore, the growing prevalence of these allergies will accelerate the phenylephrine hydrochloride market growth in the region. According to the European Federation of Allergy and Airways Diseases Patient’s Association, Allergies afflict half of all Europeans. In 2015, chronic allergic disorders affected over 150 million people in Europe. Although an estimated 45% of allergy sufferers have never been diagnosed, it is predicted that half of all Europeans will be impacted by 2025.
Phenylephrine Hydrochloride Market Players:
- Aarti Pharmalabs Limited
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- SNA Healthcare Private Limited
- Shenzhen Oriental Pharmaceutical Co., Ltd.
- Unichem Laboratories Ltd.
- Transo-Pharm Handels GmbH
- Hikma Pharmaceuticals PLC
- Eton Pharmaceuticals, Inc.
- LGM Pharma
- AstraZeneca plc
- Abbott Laboratories
There are numerous significant competitors in the fiercely competitive worldwide phenylephrine hydrochloride market. To keep a competitive edge in the market, the major industrial players employ several tactics. Among other tactics, these players are involved in strategic partnerships, mergers, acquisitions, and collaborations. One can gain important knowledge about the competitive environment of this market and strategically position themselves for success in this industry by carefully evaluating each company's phenylephrine hydrochloride market share, considering elements like product offers, production capacity, geographic reach, etc.
Recent Developments
- In December 2023, Hikma Pharmaceuticals PLC introduced Phenylephrine HCl Injection, USP, in dosages of 500 mcg/5 mL and 1,000 mcg/10 mL. Ready-to-use vials of the product have been introduced in the United States. It is recommended to raise blood pressure in people who have clinically significant hypotension, which is mostly caused by vasodilation during anesthesia.
- In October 2019, Eton Pharmaceuticals, Inc., a specialty pharmaceutical company focused on developing and commercializing innovative drug products, announced that the United States Food and Drug Administration (FDA) approved Biorphen, the first and only FDA-approved ready-to-use formulation of phenylephrine for the treatment of clinically significant hypotension caused primarily by vasodilation during anesthesia.
- Report ID: 6907
- Published Date: Dec 02, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Phenylephrine Hydrochloride Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




