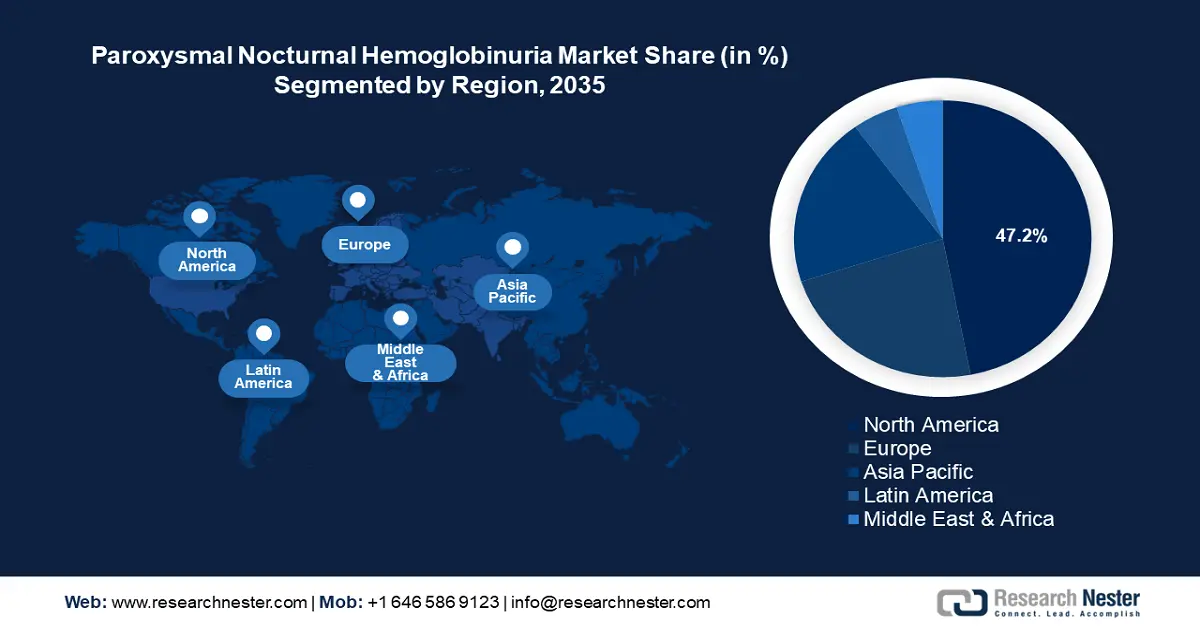
Paroxysmal Nocturnal Hemoglobinuria Treatment Market Regional Analysis:
North America Market Forecast
North America industry is anticipated to hold largest revenue share of 47.2% by 2035, owing to a high adoption rate of advanced therapies and a robust regulatory framework benefiting clinical trials and approvals of new treatments. North America is the home of major pharmaceutical companies such as Alexion Pharmaceuticals, which developed Soliris, and Ultomiris benefits the adoption in North America. Additionally, supportive regulatory programs such as the RareCare Patient Assistance Program by the National Organization for Rare Disorders reimburse registration costs for workshops, conferences, and educational programs related to rare diseases, boosting awareness of PNH treatments. The favorable trends in North America are poised to continue the robust growth of the paroxysmal nocturnal hemoglobinuria (PNH) treatment market.
The U.S. holds the largest share in the paroxysmal nocturnal hemoglobinuria treatment sector. The market’s growth in the country is owed to a robust regulatory ecosystem in the country ensuring timely approval of new therapeutics. For instance, in December 2023, McKesson Corporation announced the availability of Fabhalta (Iptacopan) by Novartis after Fabhalta was approved by the FDA in the same month. Additionally, government support of precision medicine research with programs such as the Precision Medicine Initiative creates a supportive ecosystem for advanced research on PNH. As academic institutions and pharmaceutical companies collaborate to develop gene therapies, the market is poised to maintain its profitable growth.
Canada exhibits rapid growth in the paroxysmal nocturnal hemoglobinuria (PNH) treatment market of North America. The market’s growth is attributed to growing awareness of PNH treatment and better access to advanced therapies. Canada has a publicly funded healthcare system called Medicare that makes high treatment costs of PNH affordable to patients, improving access to wide demographics. Additionally, the regulatory ecosystem in the country has been proactive in approving novel therapeutics for PNH treatment which boosts the sector in Canada. For instance, in August 2024, Voydeya was approved by Health Canada as an add-on to Ultomiris and Soliris in adults diagnosed with PNH.
Europe Market Analysis
Europe is poised to register the fastest growth in the paroxysmal nocturnal hemoglobinuria (PNH) treatment market by the end of the forecast period. The market growth is due to a strong regulatory framework and push for rare disease research benefiting the market. The European Medicines Agency (EMA) has been at the forefront by approving leading therapies for PNH treatment, ensuring availability across the region, and boosting patient access to new and advanced therapeutics. For instance, in November 2020, the European Commission provided marketing authorization to Alexion for a new formulation of Ultomiris with reduced infusion time for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). France and Germany are leading the paroxysmal nocturnal hemoglobinuria (PNH) treatment market in Europe.
Germany has a large share of the paroxysmal nocturnal hemoglobinuria sector in Europe. The market in Germany benefits from its advanced healthcare infrastructure and presence of research institutions and biotech companies advancing research on rare diseases. The favorable framework for research benefits the domestic paroxysmal nocturnal hemoglobinuria (PNH) treatment market evident by a growing number of clinical trials. For instance, in April 2023, data from phase 3 APPOINT-PNH trial was presented at the 2023 annual meeting of the European Society for Blood and Marrow Transplantation (EBMT) highlighting oral iptacopan enabling an estimated 92.2% of complement inhibitor in PNH patients and achieving 2g/dl or more hemoglobin increase without the requirement of blood transfusions.
France is positioned to register rapid growth in the paroxysmal nocturnal hemoglobinuria sector in Europe. The paroxysmal nocturnal hemoglobinuria (PNH) treatment market’s growth is owed to favorable healthcare frameworks, such as the French National Plan for Rare Diseases. The next National Plan for Rare Diseases in France will focus on research & development as per the government that is set to benefit the growth of the PNH treatment sector. Additionally, the French Registry of Marrow Failure Syndrome helps research institutions such as the American Society of Hematology to research molecular profiling for next-generation sequencing that can benefit the market’s growth. In May 2024, Aspaveli (Pegcetacoplan) was approved in Europe for the treatment of adult patients diagnosed with PNH who have hemolytic anemia. As research on PNH ramps up and a strong regulatory framework in Europe benefits the domestic paroxysmal nocturnal hemoglobinuria (PNH) treatment market in France, the PNH treatment sector is projected to continue its growth.