Nuclear Medicine Market Outlook:
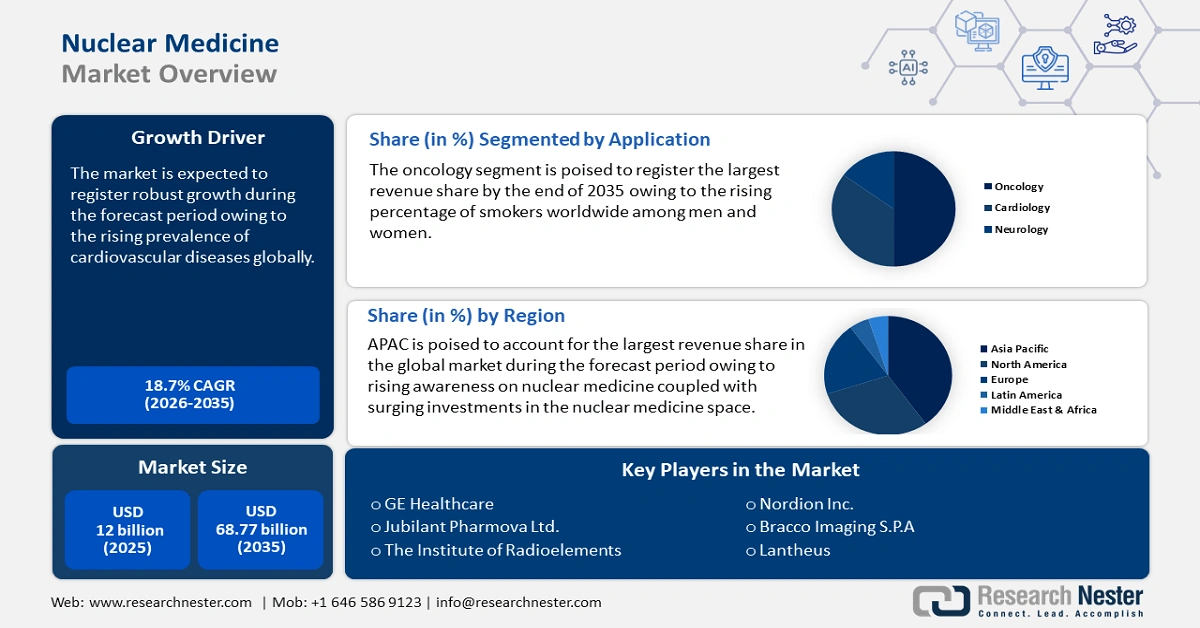
Nuclear Medicine Market size was valued at USD 12 billion in 2025 and is projected to reach USD 68.77 billion by the end of 2035, rising at a CAGR of 18.7% during the forecast period, i.e., 2026-2035. In 2026, the industry size of nuclear medicine is assessed at USD 13.97 billion.
The market is believed to expand considerably because of rising demand for personalized healthcare, continuing advances in technology, and increasing incidence of chronic disease. Major trends affecting the market include the increasing proliferation of PET and SPECT imaging technologies sufficiently to provide clinically valid diagnostic imaging information. The growth of technologies such as theranostics, which provide the capability of imaging upon injection of a targeted agent and then treating the patient with the targeted agent. The growth of radiopharmaceuticals and their use in radioligand therapy has emphasized the role of radiopharmaceuticals in treating cancer, which leverage the ability to deliver targeted radiation that destroys cancer cells while limiting damage to healthy cells. The focus on new and/or more effective early diagnosis has expanded the role of nuclear medicine in diagnosing diseases such as Alzheimer's Disease and disease processes related to the heart during the earliest stages, which may improve outcomes for patients. Finally, the market is also characterized by significant regulatory support, which includes streamlined regulatory approval for new radiopharmaceuticals and diagnostic imaging technologies.
In nuclear medicine, the momentum towards personalized medicine is particularly evident, with treatments being increasingly individualized based on a patient's genetic makeup or the particular traits of their disease. This shift relies heavily on the discovery of biomarkers, which is essential to avoiding the overtreatment of patients with therapies that will not benefit. At the same time as this is occurring, there are also signs that regulatory factors are an increasingly important ingredient to stimulate market growth. Regulatory bodies such as the FDA and EMA are working to simplify the approval pathways for new nuclear medicines, encouraging the timely integration of novel therapies and imaging technology.