Nontuberculous Mycobacteria Market Outlook:
Nontuberculous Mycobacteria Market size was over USD 9.39 billion in 2025 and is anticipated to cross USD 11.79 billion by 2035, growing at more than 2.3% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of nontuberculous mycobacteria is assessed at USD 9.58 billion.

A major driving factor for growth in the nontuberculous mycobacteria market includes the increased prevalence of NTM infection among immunocompromised and patients with chronic lung diseases. For example, in November 2024, according to the United Nations International Children's Emergency Fund (UNICEF), pneumonia is the most common infectious disease that kills over 700,000 children a year, or about 2,000 children every day. Moreover, the development of diagnosis technologies with more efficient detection capabilities of NTM species facilitates the planning of suitable treatments.
In addition, there is also rising awareness of environmental sources of NTMs, including contaminated water systems and bacteria which has led public health efforts to reduce their exposure risks. Furthermore, improved attention to these prevention strategies will probably lead to significant investments in monitoring and control measures within healthcare settings. For instance, in May 2023, the TB Alliance announced that the Cystic Fibrosis Foundation would award up to USD 3.9 million to perform preclinical testing of a compound. This may be used to treat infections brought on by a type of nontuberculous mycobacteria (NTM), which is increasingly present in individuals with cystic fibrosis.
Key Nontuberculous Mycobacteria Market Insights Summary:
Regional Highlights:
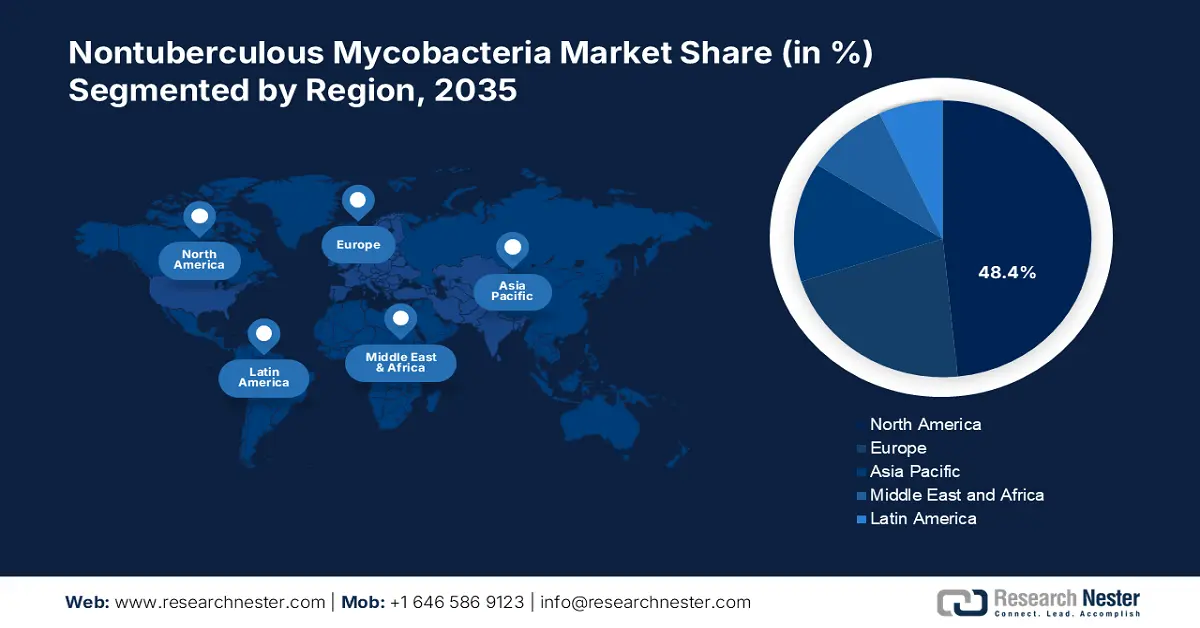
- North America leads the Nontuberculous Mycobacteria Market with a 48.4% share, fueled by the growing prevalence of chronic obstructive pulmonary disease (COPD) in the region, ensuring strong growth prospects through 2026–2035.
- The Asia Pacific nontuberculous mycobacteria market is set for robust growth through 2035, fueled by poverty, migration, population growth, and ecological changes.
Segment Insights:
- The Oral Antibiotics segment of the Nontuberculous Mycobacteria Market is expected to capture a 46.10% share by 2035, fueled by the convenience, ease of administration, and advances in oral antibiotics improving patient compliance.
Key Growth Trends:
- Increasing geriatric population
- Development of novel antimicrobial therapies
Major Challenges:
- Variability in strain pathogenicity
- High cost of treatment
- Key Players: Paratek Pharmaceuticals Inc., RedHill Biopharma Ltd., Janssen, Novartis AG, Nobelpharma Co Ltd., and more.
Global Nontuberculous Mycobacteria Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 9.39 billion
- 2026 Market Size: USD 9.58 billion
- Projected Market Size: USD 11.79 billion by 2035
- Growth Forecasts: 2.3% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (48.4% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, United Kingdom, France
- Emerging Countries: China, Japan, India, South Korea, Singapore
Last updated on : 13 August, 2025
Nontuberculous Mycobacteria Market Growth Drivers and Challenges:
Growth Drivers
- Increasing geriatric population: One significant aspect driving market expansion is the aging of the global population. An increase in the number of elderly people increases the incidence of NMT infections. It is anticipated that the prevalence of NTM infections will increase as age-related factors weaken immune systems. The need for cutting-edge medical treatments catered to the particular requirements of an aging population is underscored by this demographic trend. For instance, in October 2024, according to the UN Department of Economic and Social Affairs, by 2050, there will be 2.1 billion individuals worldwide who are 60 years of age or older, making up 26% of the total population. This is twice as many as the 1.2 billion, or 12%, in 2024.
- Development of novel antimicrobial therapies: One of the most significant growth drivers in the nontuberculous mycobacteria market is the development of novel therapies owing to the urgent need to combat rising antibiotic resistance. For instance, in May 2024, MannKind Corporation was approved by the FDA to give clofazimine inhalation suspension a fast-track designation. It gives the company a chance to step up its efforts in hopes of delivering a potentially life-changing medication to patients with NTM. This ongoing research and development effort is essential for expanding therapeutic options and fostering a more effective response to NTM infections in clinical practice.
Challenges
- Variability in strain pathogenicity: The nontuberculous mycobacteria represent a considerable challenge due to different species and strains exhibiting distinct virulence factors that influence their ability to cause disease. The mycobacterium avium complex is classically associated with chronic pulmonary infections, other species, such as mycobacterium abscessus, can produce more aggressive infections that are not easily treated. This heterogeneity makes clinical management challenging because providers may have difficulty identifying the exact NTM species causing a patient's infection given overlapping clinical presentations of other respiratory illnesses.
- High cost of treatment: The nontuberculous mycobacteria market would be constrained by the high cost of treatment, which puts a strain on patients' finances resulting in inadequate or postponed treatment, which could impair health outcomes. For instance, according to the National Library of Medicine, in 2023, the costs of NTM infection were significantly higher than those of the control group. The average cost of inpatient expenses for patients with NTM infection was USD 3934.55, whereas the average cost for the control group was USD 2997.21. In addition, the average outpatient cost for those with NTM infection was USD 2514.69, whereas the average outpatient cost for the control group was USD 2115.04.
Nontuberculous Mycobacteria Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
2.3% |
|
Base Year Market Size (2025) |
USD 9.39 billion |
|
Forecast Year Market Size (2035) |
USD 11.79 billion |
|
Regional Scope |
|
Nontuberculous Mycobacteria Market Segmentation:
Class of Drugs (Oral Antibiotics, IV Antibiotics, Anti-Nausea, Nebulized Antibiotics)
Oral antibiotics segment is set to capture over 46.1% nontuberculous mycobacteria market share by 2035. To provide convenience and ease of administration leading to improvement of patient advances in oral antibiotics continue to evolve. For instance, in January 2025, The Central Drugs Standard Control Organization (CDSCO) approved Wockhardt Limited's new oral antibiotic, Miqnaf (Nafithromycin), for the treatment of adult community-acquired bacterial pneumonia (CABP). The fight against antibiotic resistance and increased patient compliance have advanced significantly with this approval. Hence, oral antibiotics help fill the unmet therapeutic demands within the market.
Our in-depth analysis of the global nontuberculous mycobacteria market includes the following segments:
|
Class Of Drugs |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Nontuberculous Mycobacteria Market Regional Analysis:
North America Market Statistics
North America in nontuberculous mycobacteria market is poised to capture over 48.4% revenue share by 2035. The growing prevalence of chronic obstructive pulmonary disease (COPD) in the region is expected to drive future growth in the nontuberculous mycobacterial market. The hallmark of chronic obstructive pulmonary disease (COPD), a chronic respiratory disease, is the persistent and often worsening restriction of lung airflow, which increases the likelihood that NTM bacteria will infect the lungs. Thus, the need to create efficacy in treating such infections arises in the market.
Canada nontuberculous mycobacteria market is expected to attain remarkable growth during the projected timeframe. The creation of novel and efficient treatments is therefore urgently needed to satisfy the rising demand and overcome the shortcomings of existing therapies. For instance, in January 2024, Microbion Corporation declared that pravibismane has been given a second orphan drug designation for the treatment of non-tuberculous mycobacterial (NTM) infections. Hence, attaining such milestones results in elevating the landscape of treatment therapies and leads to satisfactory patient outcomes.
The U.S. nontuberculous mycobacteria market is expanding substantially attributable to the presence of prominent organizations which scrutinize and monitor the activities in the development of novel drugs. For instance, in January 2022, The Phase 2 trial of SPR720, Spero Therapeutics, Inc.'s investigational oral product candidate was developed for nontuberculous mycobacterial (NTM) disease. In addition, it was released from clinical hold by the U.S. Food and Drug Administration (FDA).
Asia Pacific Market Analysis
The nontuberculous mycobacteria market in the Asia Pacific is gaining traction and is expected to witness the fastest growth during the forecast timeline. The market is driven by factors such as poverty, migration from rural to urban areas, population growth, and adverse ecological changes. Furthermore, the rapid spread of antibiotic resistance is the primary factor causing rare infectious diseases such as nontuberculous mycobacterium infection to emerge.
China is evolving rapidly in the nontuberculous mycobacteria market owing to the development of novel drugs to treat infections and approvals to continue the innovations by companies. For instance, in January 2025, Shanghai MicuRx Pharmaceutical Co., Ltd. declared that the U.S. Food and Drug Administration (FDA) has awarded its self-developed anti-infection medication, MRX-5, Orphan Drug Designation (ODD). This is for the treatment of infections caused by non-tuberculous mycobacteria (NTM). MicuRx has achieved a major milestone in the treatment of NTM infections with this designation.
India nontuberculous mycobacteria market is witnessing notable growth due to the presence of a large pool of generic pharmaceutical manufacturers. The generic industry in the country leveraging its expertise to create affordable nontberculous treatments, making them more accessible to the patient population within the country. In addition, growing awareness about infections and increasing investment in healthcare infrastructure which fuel the market expansion.
Key Nontuberculous Mycobacteria Market Players:
- RevImmune
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Insmed
- Paratek Pharmaceuticals Inc
- RedHill Biopharma Ltd
- Janssen
- Novartis AG
- Nobelpharma Co Ltd.
Companies in the nontuberculous mycobacteria market facilitate developments in diagnostics with modern technologies that involve molecular assays to have better patient outcomes through more effective treatments and diagnostics. For instance, in October 2024, AN2 Therapeutics, Inc. announced that it had been awarded a second-year extension of a research grant from the Bill & Melinda Gates Foundation to find new small molecules that contain boron for the treatment of malaria and tuberculosis (TB). Thus, fosters innovation, accuracy, and efficacy in treatments.
Here's the list of some key players:
Recent Developments
- In April 2023, ZOSYN (piperacillin and tazobactam) injection was introduced by Baxter International Inc. It is available in Baxter's exclusive Galaxy single-dose containers, and is prescribed to treat a variety of infections brought on by susceptible bacteria.
- In January 2023, Thermo Fisher Scientific Inc. introduced the applied biosystems taqPath Seq HIV-1 Genotyping Kit, CE-IVD marked. This development increases the effectiveness of treatment, encourages the monitoring of viral resistance, and stimulates innovation in the market for infectious diseases.
- Report ID: 6954
- Published Date: Aug 13, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Nontuberculous Mycobacteria Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




