Neurotrophic Keratitis Market Outlook:
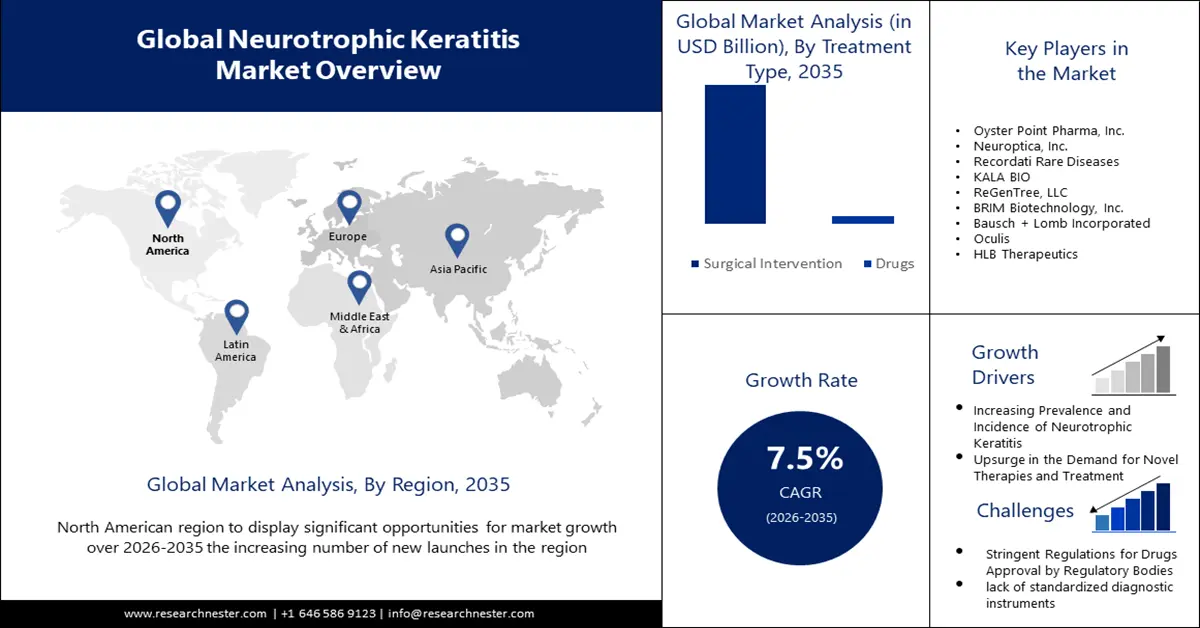
Neurotrophic Keratitis Market size was over USD 371 million in 2025 and is anticipated to cross USD 764.64 million by 2035, growing at more than 7.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of neurotrophic keratitis is assessed at USD 396.04 million.
As one age, the prevalence rate of neurotrophic keratitis rises, which is attributed to boosting the market’s growth. The World Health Organization predicts that by 2030, one in six persons will be 60 years of age or older. By this time, there will be 1.4 billion people in the world who are 60 years of age or older, up from 1 billion in 2020. The number of individuals in the globe who are 60 years of age or older will have doubled to 2.1 billion by 2050. The population of those 80 years of age and older is predicted to treble, to 426 million, between 2020 and 2050.
In addition to these, as the first medication to treat neurotrophic keratitis, a rare corneal illness, the FDA has approved Oxervate. In clinical trials, compared to 28/5 without cenegermin, 70% of patients treated with oxycontin had full corneal healing in eight weeks. Ocular hypermia, eye inflammation, eye discomfort, and increased lacrimation are typical adverse effects. Oxervate was designated as an Orphan Drug and a Priority Review drug, respectively, indicating that it could help treat a rare disease and make a major improvement. The approval for Dompe Farmaceutici SpA was given. This highlights the industry's dedication to meeting unmet medical needs by offering incentives for the development of medications aimed at uncommon diseases.