Multiple Sclerosis Drug Market - Regional Analysis
North America Market Insights
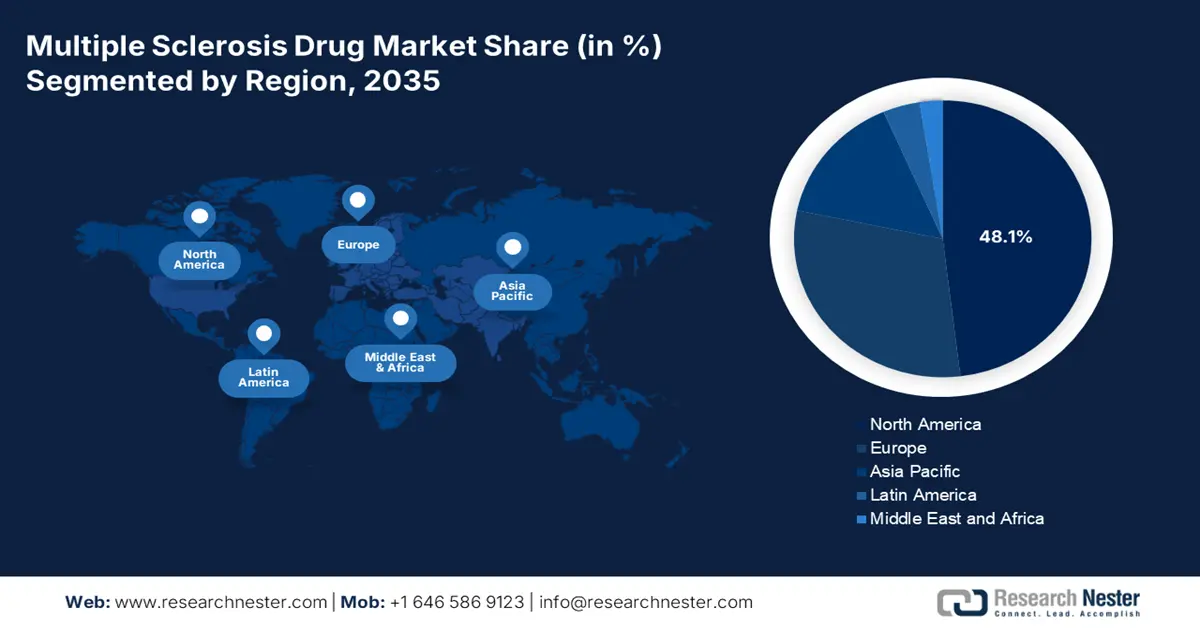
North America market is anticipated to be the dominating region, with a projected highest share of 48.1% by the end of 2035. The market’s growth in the region is subject to the U.S., readily driving the majority of the demand, further backed by a strong presence of Medicaid and Medicare coverage, along with an increase in treatment implementation rates. Besides, an expansion in telemedicine across rural areas, biosimilar adoption diminishing expenses, and the FDA’s fast track approvals are other drivers positively impacting the overall market in the region.
U.S. market is significantly growing, highly fueled by an increase in the treatment services, along with Medicaid and Medicare coverage. Additionally, Medicare Part B expenditure for monoclonal antibodies also surged, thus effectively uplifting the overall market in the country.
The multiple sclerosis drug market in Canada is also projected to grow at a rate of 6.1%, which is propelled by provincial DMT subsidies and the presence of universal health and medical coverage. Besides, Ontario’s Exceptional Access Program provides complete coverage of high-efficiency DMT expenses. Furthermore, biosimilar switching policies, indigenous health disparities, and early treatment initiation are other key trends that are skyrocketing the market in the country.
APAC Market Insights
Asia Pacific in the multiple sclerosis drug market is considered the fastest-growing region, with an expected share of 15.2% during the forecast period. The market’s development in the region is fueled by an increase in government healthcare policies, biosimilar implementation, and a surge in diagnosis volumes. China is positively impacting the region, owing to the 2023 reforms by the National Medical Products Administration (NMPA) for escalating 7 notable DMT acceptances. India is following closely through the presence of localized generics and Ayushman Bharat coverage. Then there is a generous investment by South Korea for conducting neuroprotection, while Malaysia expanded hospital and pharma collaborations, thus suitable for market upliftment in the region.
The multiple sclerosis drug market in China is gaining increased exposure with a projected revenue share of 30.5% within the forecast duration, effectively propelled by administrative policies and enhanced diagnostic rates. Together, healthcare reforms and government initiatives have been central to many aspects of the MS drug market in China. The Chinese government has made significant advancements in health care and health care funding in recent years. Moreover, there have been policy changes that improve access to treatments for chronic diseases, including MS. Healthcare insurance reforms expanded coverage for a wider variety of medications, including DMTs for MS, which gives more patients access to expensive biologics.
The multiple sclerosis drug market in India is also simultaneously growing by an expected 15% of the regional share, which is propelled by administrative policies and generic manufacturing. The Indian administration has shown sustained attention toward enhancing access to healthcare for chronic diseases like multiple sclerosis (MS). Through healthcare reforms and government-funded programs, affordable access to disease-modifying therapies (DMTs) is coming to a larger patient base. India’s National Health Policy and Ayushman Bharat (National Health Protection Scheme) are pushing for more cheap drugs for all diseases, including MS. It enables affordable access to expensive MS drugs.
Europe Market Insights
Europe is expected to hold a considerable market share of 30.2% by the end of the forecast period, catering to a 5.7% growth rate, highly fueled by biosimilar implementation as well as the increased prevalence of the disease. Germany is deliberately leading in the region, which is further driven by AMNOG pricing policies, along with an increase in the yearly growth in demand. This is followed by the UK allocating substantial part of its healthcare budget for MS therapies.
The multiple sclerosis drug market in Germany is dominating the region, with a 35% revenue share, which is propelled by an increase in biosimilar adoption and the existence of a value-specific AMNOG pricing system. Besides, the Federal Ministry of Health made the provision of a generous fund that has prioritized early high-efficiency DMTs such as Kesimpta and Ocrevus. Meanwhile, the EMA’s PRIME scheme has accelerated acceptances for 7 neuroprotection therapies between 2023 and 2025, including Merck’s evobrutinib, thus creating an optimistic outlook for the overall market.
The multiple sclerosis drug market in the UK is also growing, with a projected 25% of the region’s MS drug revenue, effectively backed by the NHS universal coverage, as well as NICE’s advanced DMT acceptances. Awareness of MS has increased within the UK population and healthcare professionals over the last few years. This is leading to earlier, more accurate diagnoses of MS. Clinicians now have accepted diagnostic approaches and technology, which means MS can be diagnosed at earlier points in the disease, where there will be a benefit to early intervention with treatments. Early intervention is likely to support long-term preventative care of MS, it is also expected to benefit treatment adherence to a routine medication.