Molecular Transport Medium Market Outlook:
Molecular Transport Medium Market size was valued at USD 1.04 billion in 2025 and is set to exceed USD 1.17 billion by 2035, registering over 1.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of molecular transport medium is estimated at USD 1.05 billion.
The growth of the market can be attributed to the rising number of experiments at labs and research institutions. The world was being prepared for pandemics for a prolonged time where countless clinical trials were being conducted to develop treatment and diagnosis solution for infectious diseases. The percentage of research and development expenditure (in % GDP) was 2.63% in 2020, up from 2.13% in 2017. Government of various nation are spending notable sum on the development of molecular transportation medium since it is the best medium to gather medical samples without endangering the lives of healthcare providers.
In addition to these, global molecular transport medium market trends such as, rowing adoption of transport mediums for infectious disorders and rising awareness regarding the early detection of viral diseases compelling governments to initiate management measures to prevent outbreaks of these infections are further expected to influence the market growth positively over the forecast period. The U.S. funding for global health security for the fiscal year 2021 was estimated to reach approximately USD 10 billion up from around USD 1 billion in 2001-11. Also, additional funding was provided for the emergency outbreaks of Ebola and the Zika virus. Hence, all these factors are estimated to propel the market growth over the forecast period.
Key Molecular Transport Medium Market Insights Summary:
Regional Highlights:
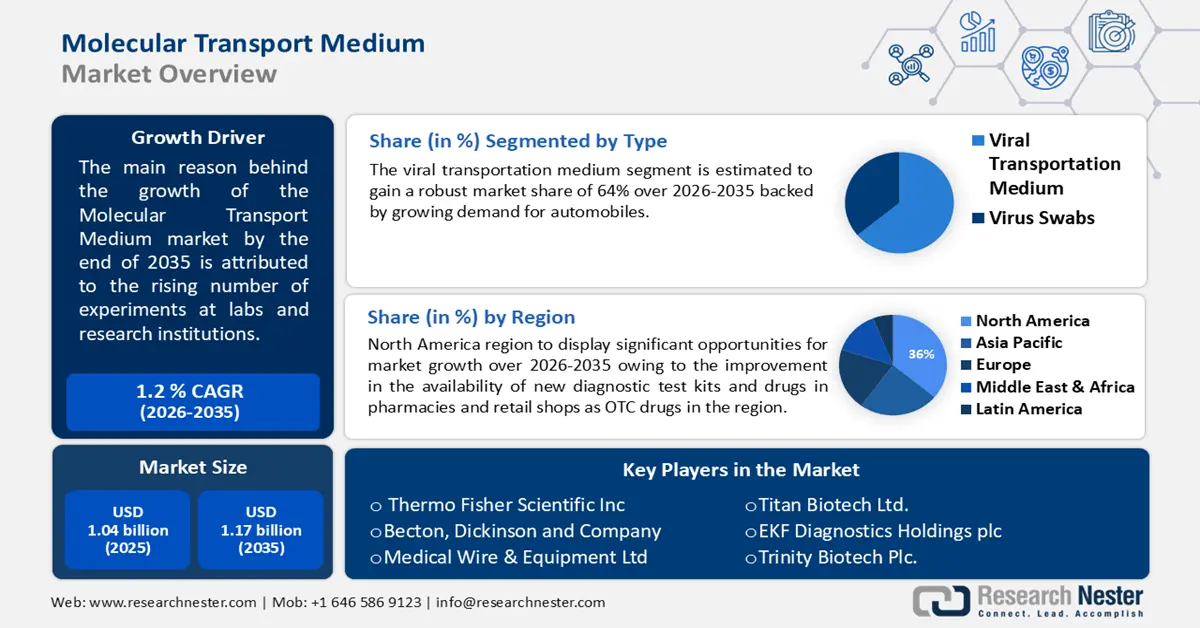
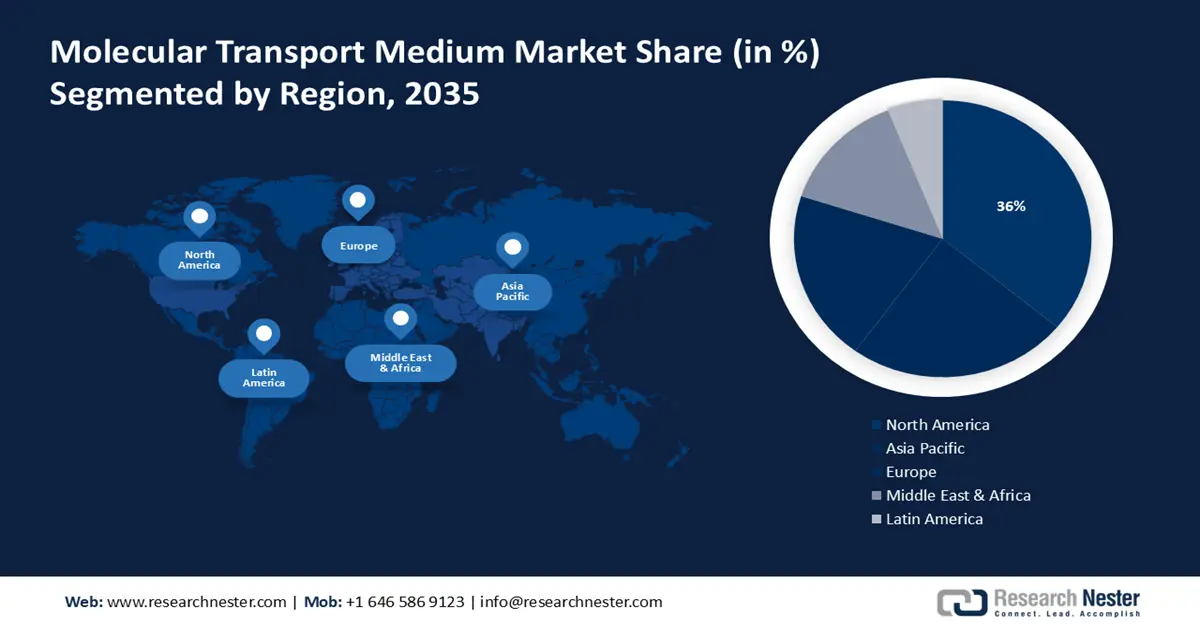
- North America is anticipated to command a 36% share of the molecular transport medium market by 2035 owing to the wider availability of new diagnostic kits and OTC drugs.
- The Asia Pacific region is projected to expand significantly by 2035 impelled by the rising burden of infectious diseases and a growing geriatric population.
Segment Insights:
- The viral transportation medium segment in the molecular transport medium market is projected to secure the largest share by 2035 propelled by the surge in demand following the COVID-19 outbreak and the development of advanced contamination-preventive kits.
- The diagnostic laboratories segment is anticipated to command a substantial share by 2035 supported by the rising volume of clinical trials and diagnostic testing driven by increasing awareness of early diagnosis.
Key Growth Trends:
- Rise in the Number of Infectious Diseases Worldwide
- Increasing Number of Diagnostic Tests
Major Challenges:
- Unpredictable Nature of Seasonal Infections
- High Risk of False Results by Molecular Medium
Key Players: Thermo Fisher Scientific Inc, Becton, Dickinson and Company, Medical Wire & Equipment Ltd, Titan Biotech Ltd., EKF Diagnostics Holdings plc, Trinity Biotech Plc., Puritan Medical Products Company LLC, Longhorn Vaccines and Diagnostics, LLC, Mylab Discovery Solutions Pvt. Ltd., 2B Scientific Limited.
Global Molecular Transport Medium Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.04 billion
- 2026 Market Size: USD 1.05 billion
- Projected Market Size: USD 1.17 billion by 2035
- Growth Forecasts: 1.2%
Key Regional Dynamics:
- Largest Region: North America (36% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Germany, United Kingdom, Japan
- Emerging Countries: India, Brazil, South Korea, Singapore, Mexico
Last updated on : 21 November, 2025
Molecular Transport Medium Market - Growth Drivers and Challenges
Growth Drivers
- Rise in the Number of Infectious Diseases Worldwide - According to WHO, the number of infectious and parasitic diseases touched 7.2 million in 2018 globally. Furthermore, in 2019, the number of cases of various infectious disease was estimated to rise globally such as, tuberculosis (8000), meningococcal disease (400), salmonella (55,000), and Lyme disease (35,000).Molecular transport medium is most suitable for the diagnosis of infectious diseases since it prevents the spreading of infection among the healthcare staff. MTM are known to preserve the sample to its initial state by keeping them close as mush as required. This process becomes helpful in preventing the spread of viruses in the environment for days and weeks. Hence, this factor is projected to hike the market growth over the forecast period.
- Increasing Number of Diagnostic Tests - More than 3 billion Vitro diagnostic tests are conducted every year (both FDA-reviewed and lab-developed tests) across the globe.
- Rise In Experiments in Laboratories and Research Institutes - According to the data by the World Bank, there were 1597 researchers involved in research and development activities per 1,000,000 people, as of 2018, up from 1412 per million in 2015.
- Rising Awareness for the Health of Co-Workers in a Laboratory - Total fatal occupational injuries in healthcare practitioners and technical occupations decreased from 60 in 2016 to 51 in 2020, and healthcare support occupations raised from 30 in 2016 to 44 in 2020. Molecular transport medium is the best solution to prevent the spread of infection in co-workers and owing to this, healthcare providers highly prefer MTMs.
- Growing Expenditure for R&D Activities – based on the data released by the World Bank, the health expenditure for research and development was stated to reach 2.63% up from 2.2% in 2028. Research and development of molecular transport medium is at its peak and growing health expenditure has been proved to be very beneficial for it.
Challenges
- Unpredictable Nature of Seasonal Infections - There are multiple infectious diseases that spread in global population and their nature of spreading and affecting the can be quite unpredictable. This condition makes the molecular transport medium as ordinary as other mediums are. Hence, this factor can limit the market growth over the forecast period.
- High Risk of False Results by Molecular Medium
- Uncertain Occurrence of Infections Leading to the Adoption of a Conservative Approach
Molecular Transport Medium Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
1.2% |
|
Base Year Market Size (2025) |
USD 1.04 billion |
|
Forecast Year Market Size (2035) |
USD 1.17 billion |
|
Regional Scope |
|
Molecular Transport Medium Market Segmentation:
Type Segment Analysis
The global molecular transport medium market is segmented and analyzed for demand and supply by type into viral transportation medium and virus swabs. Between these two types, the viral transportation medium segment is estimated to gain the largest market share over the projected time frame. The growth of the segment can be attributed to the sudden break out of the COVID-19 pandemic triggering the demand for transport medium products and kits worldwide. According to the Journal of Clinical Microbiology between 3000 to 5000 viral transport medium tubes are produced each day to support SARS-CoV-2 PCR testing globally. The innovation of kits containing vials, double-ended flocked swabs, antibiotics, and other antibacterial and antifungal items that prevent contamination is estimated to drive the segment growth.
Application Segment Analysis
The global molecular transport medium market is also segmented and analyzed for demand and supply by application into diagnostic laboratories, microbiology laboratories, hospitals & clinics, and others. Amongst these three segments, the diagnostic laboratories segment is expected to garner a significant share. The growth of this segment can be attributed to the surge in clinical trials and diagnostic testes backed by the rising awareness of early diagnosis in the global population. The higher prevalence of severe infectious diseases across the globe has spurred the demand for molecular transport medium worldwide. It was observed that in the United States, over 6 billion diagnostic tests are performed annually.
Our in-depth analysis of the global market includes the following segments:
|
By Type |
|
|
By Application |
|
|
By Indication |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Molecular Transport Medium Market - Regional Analysis
North American Market Insights
North America industry is expected to dominate majority revenue share of 36% by 2035. The growth of the market can be attributed majorly to the improvement in the availability of new diagnostic test kits and drugs in pharmacies and retail shops as OTC drugs. As of December 2021, approximately 300 million at-home rapid test kits were available in the market in the U.S. which reached nearly 24 million in August 2021. Additionally, the rise in the number of infections, parasitic diseases coupled with increase in the manufacturing capacity of vaccines and drugs is estimated to boost the regional market growth during the forecast period. For instance, COVID-19 vaccine manufacturing was estimated to hit around 7 million doses in 2021 with the production rate of around 1 billion doses per month by vaccine manufacturers across the world. The recall was done to reduce the false positive test results, unnecessary quarantine, and treatment with antivirals.
APAC Market Insights
Furthermore, the market in the Asia Pacific is also estimated to witness noteworthy growth opportunity over the forecast period. Rising cases of infectious diseases such as, COVID-19, Lyme Disease, TB, and others backed by the boom in the population along with significant number of geriatrics in the region are estimated to be major factors to expand the market size over the forecast period. In Asia Pacific, preventable infections are considered to be one of the major causes of death that is anticipated to boost the demand for molecular transport medium for collecting samples. Therefore, all these factors are anticipated to boost the market growth over the forecast period.
Molecular Transport Medium Market Players:
- Thermo Fisher Scientific Inc
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Becton, Dickinson and Company
- Medical Wire & Equipment Ltd
- Titan Biotech Ltd.
- EKF Diagnostics Holdings plc
- Trinity Biotech Plc.
- Puritan Medical Products Company LLC
- Longhorn Vaccines and Diagnostics, LLC
- Mylab Discovery Solutions Pvt. Ltd.
- 2B Scientific Limited
Recent Developments
-
Thermo Fisher Scientific Inc is offering In Vitro Diagnostic Requirements (IVDR) versions to help molecular diagnostic manufacturers to adopt higher standards. The demand for IVDR is growing on account of growing awareness for quick clinically relevant Oncology biomarkers identification and in the diagnosis of multiple-infectious pathogens. It is going to be huge help for healthcare providers to make serious treatment decisions associated with infection diseases, cancer, and others.
-
Becton, Dickinson and Company to introduce a new high throughout molecular diagnostic combination test, BD MAX, for SARS-CoV-2 and Influenza A/B, the second test for BD COR have been CE marked to the IVD directive. In this test, a single nasopharyngeal swab sample are used to identify if the patients have flu, COVID-19, or RSV.
- Report ID: 4251
- Published Date: Nov 21, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Molecular Transport Medium Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




