Microbiology Testing Market - Regional Analysis
North America Market Insights
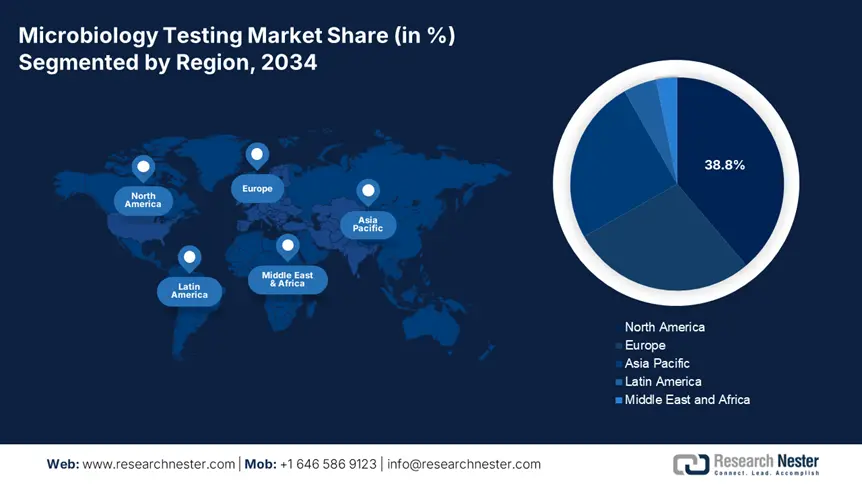
North America is anticipated to capture the highest share of 38.8% in the global microbiology testing market during the discussed tenure. The amplifying burden of healthcare-associated infections, including 1.8 million annual Clostridioides difficile cases till 2024, is fueling demand for advanced toxin detection kits, as reported by the CDC. The region's proprietorship is further solidified by stringent FDA and CLIA standards that ensure testing accuracy and reliability. Thus, robust expansion of CLIA-certified laboratories across North America enables widespread acceptance of innovative diagnostic solutions, such as PCR and other microbiology diagnoses.
The U.S. dominates the regional microbiology testing market, which is primarily backed by a high occurrence rate of hospital-acquired infections, accounting for over 750,007 annual cases till 2024, as per the CDC report. Besides, in 2024, Medicare drafted an outlay of $2.4 billion budget for sepsis bundle payments by 2030, according to the Centers for Medicare & Medicaid Services (CMS). The U.S. also maintains a strong emphasis on global trade by importing $1.6 billion worth of testing devices, while exporting $850.7 million of diagnostic instruments, as observed by the U.S. International Trade Commission (USITC).
Canada is poised to expand remarkably in the microbiology testing market with financial support from public investments. This can be exemplified by the $340.6 million federal AMR action plan, enacted by the Public Health Agency of Canada (PHAC) in 2023. Despite the challenges from Health Canada's lengthy approval timelines, the innovation aspect of the nation continues to thrive with the $50.5 million biotech R&D fund from the Johnson & Johnson Innovation JLABS - Toronto. These allocations position Canada as a growing but carefully regulated market within the regional territory in this category.
APAC Market Insights
Asia Pacific is predicted to position itself as the fastest-growing region in the global microbiology testing market by the end of 2034. The increasing infectious disease burdens and healthcare infrastructure development are the two major growth engines in this region. For instance, the National Institute of Infectious Diseases (NIID) recorded an annual 15.6% rise in HAI cases in Japan, while South Korea increased its bio-health budget by 25.8% in 2024, as per the Ministry of Science and ICT (MSIT). On the other hand, the count of eligible patients in Malaysia doubled from 2013 to 2024, demonstrating the region's accelerating diagnostic needs.
China is commanding regional dominance over the APAC microbiology testing market with 40.5% revenue share. This leadership is accomplished by its aggressive TB elimination initiatives and comprehensive HIV screening programs. For instance, in 2024, the National Medical Products Administration (NMPA) allocated $3.1 billion in funding to support these critical public health efforts. This is not only fueling substantial demand but also encouraging both domestic and foreign biotech pioneers to invest in this sector. Moreover, targeted government investments continue to position China as the largest and most influential market across the region.
India represents itself as one of the epicenters of global-scale commercial expansion in the microbiology testing market. Testifying to the same, in 2024, the WHO underscored a 200.7% surge in demand for assessments related to this category since 2010. This demographic enlargement is primarily followed by government-backed health campaigns and Ayushman Bharat-initiated lab network expansion program, as unveiled by the MoHFW. The country also fosters lucrative opportunities curated from affordability gaps, where approximately 60.6% of patients pay $25.4-$50.3 per test, according to the 2024 WHO survey. This prompted the cultivation of innovative collaboration models that are improving access.
Adoption Rates of Microbiology Testing
|
Country |
2022 Adoption Rate |
2023 Adoption Rate |
2024 Adoption Rate |
2025 (Projected) |
|
Australia |
63.4% |
68.8% |
74.4% |
80.8% |
|
South Korea |
52.3% |
60.4% |
67.7% |
75.4% |
|
Malaysia |
38.3% |
45.6% |
53.4% |
60.3% |
Source: Health Ministry of the Respective Countries
Europe Market Insights
The Europe microbiology testing market is expected to maintain a steady growth during the timeline between 2025 and 2034. The rising AMR threats, aging populations, and stringent regulations are collectively propelling demand in this sector. In this regard, the WHO revealed that, from 2020 to 2024, TB prevalence increased by 9.8% in the eastern zone of the region. On the other hand, the governing body of France allocated €2.1 billion to escalate development and deployment in this category. Furthermore, the €2.8 billion Health Data Space initiative is standardizing diagnostics across borders, which further prompted modernization in this category all over Spain and Italy, cutting processing times by 20.4%, as per the Italian Medicines Agency (AIFA).
Germany is expected to lead the Europe microbiology testing market with a 30.8% revenue share by 2034. The regional dominance of the country is consolidated by tightening regulations and diagnostic innovations. For instance, in 2024, the G-BA mandated 100.5% sterility testing of implants, which further created a €1.6 billion microbial QC opportunity, as displayed by a report from the Federal Ministry of Health (BMG). However, the country's dual-trial requirements add €2.5 million in development costs for each test. Thus, technological breakthroughs, such as Qiagen's AI-powered PCR systems, are minimizing operational expenses in laboratories.
The UK is poised to maintain a strong captivity with a 25.5% share in the Europe microbiology testing market. In support of this augmentation, in 2024, the National Health Service (NHS) allocated a £2.4 billion annual fund to sepsis bundle payments and £500.5 million as AMR research investment, according to the Department of Health and Social Care (DHSC). The country also witnessed notable diagnostic improvements, including a 25.5% reduction in misdiagnosed UTIs following PCR test implementation in primary care in 2023. This is encouraging both patients and medical settings to invest in this category.
Adoption Rates of Microbiology Testing (2021-2025)
|
Country |
2021 Adoption Rate |
2023 Adoption Rate |
2025 (Projected) |
Key Driver |
|
Spain |
42.4% |
51.5% |
60.6% |
EU AMR Action Plan (2022) |
|
Italy |
38.4% |
47.3% |
58.3% |
AIFA Lab Modernization Fund |
|
Russia |
28.6% |
35.8% |
45.7% |
National Pharma 2030 Strategy |
Source: National health agencies and EU AMR Surveillance Network