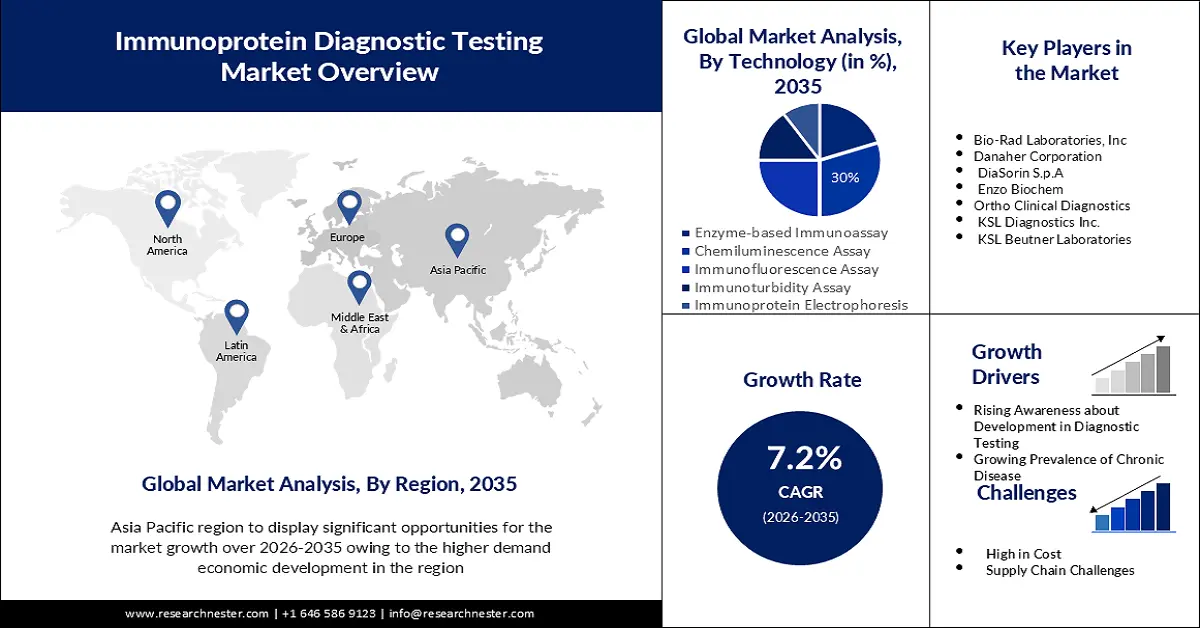
Immunoprotein Diagnostic Testing Market Outlook:
Immunoprotein Diagnostic Testing Market size was valued at USD 9.52 billion in 2025 and is set to exceed USD 19.08 billion by 2035, registering over 7.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of immunoprotein diagnostic testing is estimated at USD 10.14 billion.
The growth of this market can be attributed to the growing presence of infectious and chronic diseases. Products for immunoprotein diagnostic testing have increased in popularity due to the high frequency of chronic diseases like infectious diseases, cardiovascular diseases, cancer, obesity, and rheumatoid arthritis. Rapid diagnosis is urgently required due to the chronic diseases' rising mortality rates. Nearly half of all fatalities globally are thought to be caused by infectious diseases like AIDS, malaria, and tuberculosis, according to the Centers for Disease Control and Prevention (CDC).
Chemiluminescence assays have been developed as a result of technological developments in immunoprotein diagnostics. This technique uses fully automated devices and high throughput to assist in the quick detection of chronic diseases. Chemiluminescence tests only require a brief processing period, often between 30 and 45 minutes. This technology allows for on-board reagent capacity to speed up turnaround time and reduces any system workflow interruptions. Additionally, the launch of a wide range of goods has been facilitated by the fusion of complementing laboratory technologies with technological advancements related to immunological reagents and kits.