Hidradenitis Suppurativa Market Outlook:
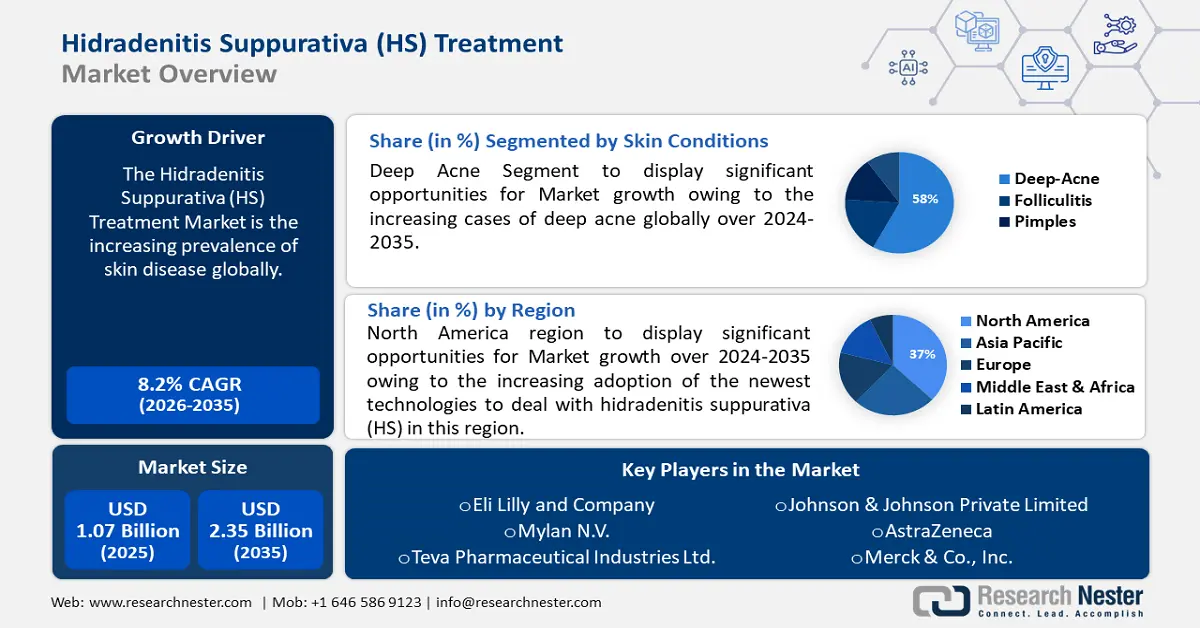
Hidradenitis Suppurativa Market size was valued at USD 1.07 billion in 2025 and is likely to cross USD 2.35 billion by 2035, registering more than 8.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of hidradenitis suppurativa is assessed at USD 1.15 billion.
The increasing prevalence of skin disease globally will exponentially help the hidradenitis suppurativa market to grow in the expected CAGR. Between 1990 and 2019, there was a 106%, 80%, and 104% increase in the number of new cases, DALYs, and fatalities from bacterial skin illnesses related to decubitus ulcers. The accuracy of the information available to characterize the prevalence of skin diseases in developing nations is still questionable. Our goal was to investigate the frequency of skin disorders and their disability-adjusted life years (DALYs) in the general Thai population in Ubonratchathani.
Another reason to propel the hidradenitis suppurativa market by the end of 2036 is the rising frequency of chronic diseases across the world. The yearly rate of DALYs per 100,000 persons is displayed on this map. This allows it to measure how the global burden of both illness and mortality is distributed. In some of the healthiest areas of the country, the 2019 DALY rate was less than 20,000 per 100,000 people. In contrast, the rate was many times greater in the poorest areas—above 60,000 in many African countries. Millions of people globally endure greatly from infectious diseases, but another public health problem is getting progressive attention: chronic noncommunicable diseases (NCDs), such as diabetes and heart disease, are turning out to be one of the biggest problems to public health. The huge impact that non-communicable diseases (NCDs) have on individual health also impacts the quality of life for those who endure these situations, along with for those who care for them.
Key Hidradenitis Suppurativa Market Insights Summary:
Regional Highlights:
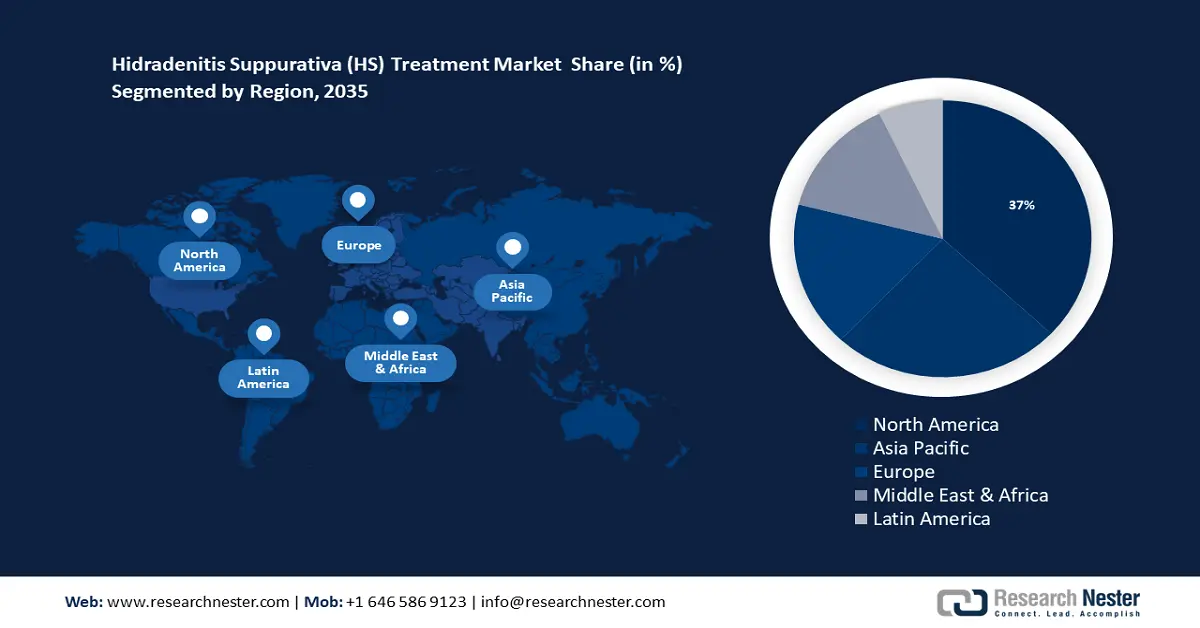
- North America hidradenitis suppurativa market is expected to capture 37% share by 2035, driven by the increasing adoption of the newest technologies to address hidradenitis suppurativa.
- Asia Pacific market is poised for huge growth from 2026 to 2035, fueled by the increasing frequency of obesity, particularly in China, driving demand for hidradenitis suppurativa treatments.
Segment Insights:
- The deep acne segment in the hidradenitis suppurativa market is expected to witness substantial growth through 2035, attributed to the rising number of deep acne cases globally, particularly among adolescents.
- Medications segment in the hidradenitis suppurativa market is projected to achieve 40% growth by the forecast year 2035, fueled by the increasing global use of medications to treat acne.
Key Growth Trends:
- The Rising Adoption of Pipeline Treatments Globally
- Rising Investment in Healthcare Infrastructure Worldwide
Major Challenges:
- The Excessive Cost Related to Hidradenitis Suppurativa
- Inattentiveness of Disease to Physicians
Key Players: F. Hoffmann-La Roche Ltd., Business PlanningMain Product OfferingsFinancial ExecutionMain Performance IndicatorsEli Lilly and Company, Mylan N.V., Teva Pharmaceutical Industries Ltd., Johnson & Johnson Private Limited, AstraZeneca, Merck & Co., Inc., Bayer AG, AbbVie Inc., Abbott.
Global Hidradenitis Suppurativa Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.07 billion
- 2026 Market Size: USD 1.15 billion
- Projected Market Size: USD 2.35 billion by 2035
- Growth Forecasts: 8.2% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (37% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, China, France
- Emerging Countries: China, India, Japan, South Korea, Malaysia
Last updated on : 17 September, 2025
Hidradenitis Suppurativa Market Growth Drivers and Challenges:
Growth Drivers
- The Rising Adoption of Pipeline Treatments Globally - For many years, there has been unequal access to and quality of healthcare around the globe. Despite calls for change from payers, providers, and patients alike, the healthcare industry has generally resisted outside interference. Thus, we need to alter our way of thinking before we can promote transformation. The conventional linear pipeline-service-model way of thinking needs to give way to a platform approach, which unites producers, consumers, and longitudinal medical data to jointly develop a new healthcare paradigm that radically transforms the way we deliver care and progress treatments. Through the development of new, thoroughly tested end-to-end algorithms and other creative ways to improve care, a healthcare platform will enable the aggregation and harmonization of disparate clinical and non-clinical data, enabling us to treat more patients, connect patients and data to create new knowledge and transform the health care industry.
- Rising Investment in Healthcare Infrastructure Worldwide - The ongoing pandemic is still requiring the attention and resources of healthcare systems, but the global healthcare industry is rising to the challenge. They keep improving the quality of life for their employees by changing the nature, scope, and location of work, quickly expanding patient access to virtual health services, and forming alliances to manufacture and acquire the necessary medications, therapies, and supplies. Prior research conducted by the OECD Health Division has demonstrated the significant opportunity to reduce unnecessary spending and achieve efficiency improvements within the health system. In the medium to long term, the overall rises in health spending may be lessened by a combination of targeted spending and initiatives to cut unnecessary spending.
- Rising Public and Private Initiatives - For those with mild cases of hidradenitis suppurativa, hormone tablets, such as estrogen-containing combined oral contraceptives like estradiol and estradiol/norgestimate, may be helpful. Spironolactone is frequently used to lessen the need for isotretinoin, a medication primarily used to treat acne and antibiotics. Sometimes hidradenitis suppurativa is treated with isotretinoin.
Challenges
- The Excessive Cost Related to Hidradenitis Suppurativa - According to a study published online ahead of print in the American Journal of Clinical Dermatology, healthcare costs are high among adults and adolescents in the US who have hidradenitis suppurativa (HS), and the comorbidity burden in patients with the chronic skin condition appears to expand over time. Not only are HS incidence rates rising, but patients' options for therapy are also limited because adalimumab is the only biologic that has been licensed to treat HS. Garg and colleagues assessed the sociodemographic, clinical, and socioeconomic features of HS patients during three years to better manage this illness.
- The findings revealed that whereas 68.7% of adults had commercial insurance and 31.3% had Medicare, all adolescents were covered by commercial insurance. There is little and inconsistent data on the costs associated with hidradenitis suppurativa (HS), however, HS does result in substantial costs.
- Inattentiveness of Disease to Physicians
- Lack of Competent Professionals
Hidradenitis Suppurativa Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
8.2% |
|
Base Year Market Size (2025) |
USD 1.07 billion |
|
Forecast Year Market Size (2035) |
USD 2.35 billion |
|
Regional Scope |
|
Hidradenitis Suppurativa Market Segmentation:
Skin Condition Segment Analysis
The deep acne segment will grow the most by the forecast period and will hold almost 58% in the hidradenitis suppurativa market because of the increasing cases of deep acne globally. For instance, with up to 50 million cases every year, acne is the most prevalent skin ailment in the US. Eighty-five percent of those in the 12 to 24 age range have some form of acne. One of the most frequent skin conditions in the globe, acne vulgaris is pretty upsetting to patients. The majority of acne sufferers also experience social disengagement and low self-esteem. The purpose of this study was to determine how common acne was among medical students and how it affected their quality of life. It assesses the patterns of self-treatment utilization as well. Acne in adolescents has been linked to high glycemic load diets and increased milk consumption, according to several research. This may be explained by the abundance of insulin-like growth factor (IGF) and the hormones found naturally in milk.
Treatment Type Segment Analysis
The medications segment will have superior growth during the forecast period and will hold around 40% of the revenue share of the hidradenitis suppurativa market owing to the increasing uses of medications globally to cure acne worldwide. Dermatology devices are becoming more and more important across the world. Patients with acne suffer greatly regardless of the severity of the condition. According to the dermatologists’ panel, addressing environmental elements that are significant for the person with acne may aid in education, prevention, efficient management, and maintenance of acne. They concurred that due to their aging skin and social surroundings, adult female acne sufferers have particular needs. The effectiveness and tolerability of prescription acne treatments may be balanced by nonprescription acne treatment items.
Our in-depth analysis of the global hidradenitis suppurativa market includes the following segments:
|
Clinical Stage |
|
|
Skin Conditions |
|
|
Treatment Type |
|
|
Route of Administration |
|
|
Medication Types |
|
|
End-Users Industries |
|
|
Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Hidradenitis Suppurativa Market Regional Analysis:
North American Market Insights
The hidradenitis suppurativa market in the North America region will have the biggest growth during the forecast period with a revenue share of around 37%. This growth will be noticed owing to the increasing adoption of the newest technologies to deal with hidradenitis suppurativa (HS) in this region. For instance, on October 31, 2023, Novartis A.G., an international leader in immuno-dermatology and rheumatology, declared that the US Food and Drug Administration (FDA) has accepted Cosentyx® (secukinumab) to cure lessen to massive hidradenitis suppurativa (HS) in adults. Cosentyx is the only FDA-licensed completely human biologic that straight inhibits interleukin-17A (IL-17A), a cytokine believed to be involved in the inflammation of HS.
APAC Market Analysis Insights
The hidradenitis suppurativa market in the APAC region will also encounter huge growth during the forecast period and will hold the second position owing to the increasing frequency of obesity in this region. Although they are typically even lower, the corresponding numbers in the Eastern Hemisphere—primarily Asia—seem to be improving. Ma et al. provide strong evidence in this journal issue that China is seeing a very concerning rise in obesity, especially abdominal obesity. Given the magnitude of China's population and this growth, we are probably seeing an unprecedented development, at least to an extent.
Hidradenitis Suppurativa Market Players:
- F. Hoffmann-La Roche Ltd.
- Company Overview
- Business Planning
- Main Product Offerings
- Financial Execution
- Main Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Eli Lilly and Company
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Johnson & Johnson Private Limited
- AstraZeneca
- Merck & Co., Inc.
- Bayer AG
- AbbVie Inc.
- Abbott
Recent Developments
March 26, 2024: F. Hoffmann-La Roche Ltd., declared the U.S. Food and Drug Administration (FDA) acceptance of the cobas® Malaria test for utilization on the cobas® 6800/8800 Systems. This accepted test can help healthcare professionals in limiting the possible risks of patient infection from imbued blood materials. The cobas Malaria test gives a highly sensitive and particular solution to assist confirm that polluted blood units are eliminated from the blood supply.
March 05, 2024: F. Hoffmann-La Roche Ltd. and Alnylam declared that the Phase II KARDIA-2 study [NCT05103332] of zilebesiran, an examinational RNAi therapeutic in growth for the therapy of hypertension (high blood pressure) - the foremost cause of cardiovascular disease globally - met its basic endpoint. People with mild to intermediate hypertension cured with zilebesiran added to standard-of-care hypertension medicines experienced a clinically and numerically substantial limitation in systolic blood pressure at month three.
- Report ID: 5990
- Published Date: Sep 17, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Hidradenitis Suppurativa Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




