Hepatitis E Diagnostic Tests Market Outlook:
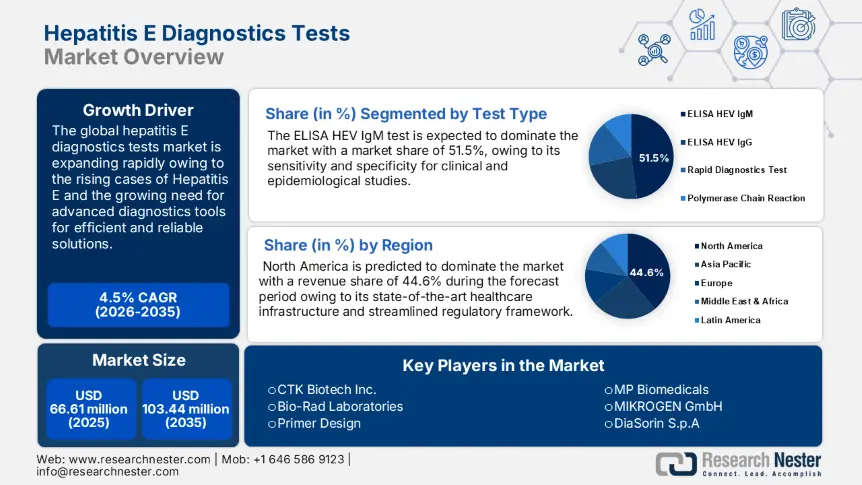
Hepatitis E Diagnostic Tests Market size was over USD 66.61 million in 2025 and is projected to reach USD 103.44 million by 2035, growing at around 4.5% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of hepatitis E diagnostic tests is evaluated at USD 69.31 million.
With high infection incidence of hepatitis E, increasing infection levels, especially in developing regions, and a growing demand for prompt and accurate methods of diagnosis, the hepatitis E diagnostic tests market is witnessing a surge. For instance, the World Health Organization (WHO), estimates 20 million HEV infections worldwide every year, leading to an average of 3.3 million symptomatic cases of HEV infection.
Moreover, improved PCR techniques, point-of-care testing devices for diagnosing hepatitis E virus infections easily, and other advanced serological and molecular testing are transforming the diagnostic landscape. Key players such as Roche, Abbott Laboratories, and Bio-Rad Laboratories hold significant hepatitis E diagnostic tests market shares and continue research and development by investing in developing better and more cost-effective diagnostic solutions. All these early detection and management alternatives with digital health technologies including mobile health applications are making the market foothold a strong presence and growth.
Key Hepatitis E Diagnostic Tests Market Insights Summary:
Regional Highlights:
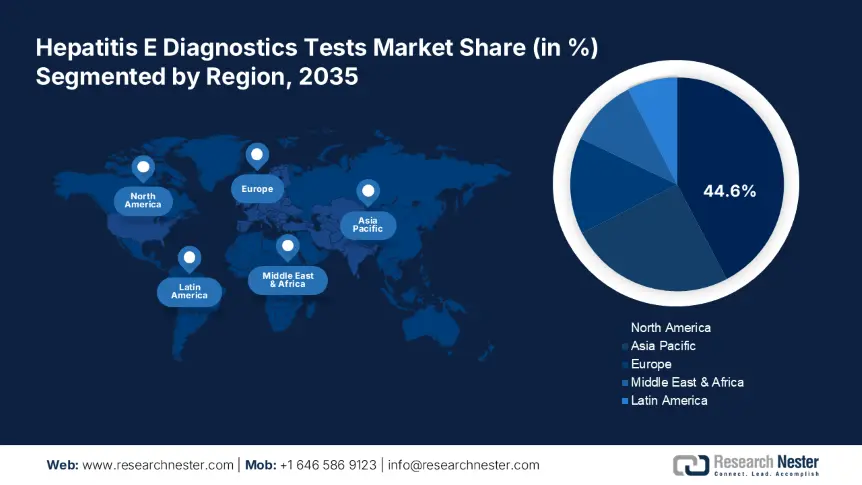
- North America's 44.6% share in the Hepatitis E Diagnostic Tests Market is driven by well-developed health infrastructure, research funding, and robust regulatory frameworks, positioning it as a leader in diagnostic advancements through 2035.
- The Asia Pacific region is poised for the fastest growth in the Hepatitis E Diagnostic Tests Market from 2026 to 2035, driven by rapid urbanization, expanding middle-income groups, and technological advancements.
Segment Insights:
- The Blood segment is expected to see lucrative growth by 2035, fueled by the increasing need for reliable molecular diagnostic techniques.
- The ELISA HEV IgM Test segment is projected to capture 51.5% market share by 2035, driven by its high sensitivity, specificity, and ease of use in diverse healthcare settings.
Key Growth Trends:
- Surging incidents of Hepatitis E
- Innovative diagnostic technologies
Major Challenges:
- Lack of standardized diagnostic tools
- Cost of advanced diagnostic
- Key Players: Fortress Diagnostic, Genscript Biotech Corporation, Guangzhou Wondfo Biotech Co., Ltd., Medsource Ozone Biomedicals Pvt. Ltd., MIKROGEN GmbH, MP Biomedicals.
Global Hepatitis E Diagnostic Tests Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 66.61 million
- 2026 Market Size: USD 69.31 million
- Projected Market Size: USD 103.44 million by 2035
- Growth Forecasts: 4.5% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (44.6% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, China, Germany, Japan, United Kingdom
- Emerging Countries: India, China, Brazil, Russia, Mexico
Last updated on : 12 August, 2025
Hepatitis E Diagnostic Tests Market Growth Drivers and Challenges:
Growth drivers
- Surging incidents of Hepatitis E: Poor sanitation and contaminated water together with increased global travel have been contributing factors to the increasing incidence of hepatitis E. This viral infection is transmitted mainly via the fecal-oral route and is particularly prevalent in areas with substandard water management. An increase in hepatitis E has also led to a growth in the demand for more sensitive diagnostic equipment. The key growth factors include the advancement of molecular diagnostic technology, public health efforts in early detection, and a better understanding of the prevention and treatment of hepatitis E. Such factors are vital in fighting and controlling the spread of the virus worldwide.
- Innovative diagnostic technologies: The hepatitis E diagnostic tests market has witnessed tremendous growth due to advances and innovation in technologies for enhancing detection with improved sensitivity. Recent improvements include the integration of molecular techniques such as PCR that enable rapid detection of HEV RNA in clinical samples. Serological assays, using new ELISA platforms, further increase sensitivity and specificity in the detection of anti-HEV antibodies, thus facilitating earlier diagnosis and epidemiologic research and fueling market growth. These factors collectively contribute toward more effective surveillance and management of hepatitis E as part of addressing public health challenges emanating from the disease and driving the growth of the hepatitis E diagnostic tests market.
- Increasing awareness: Improved awareness of hepatitis E and wide-ranging screening programs are the main factors in managing the virus. Rising public health campaigns to educate populations of causes and ways in which the disease may be prevented have boosted the demand for early diagnosis testing. Combining such efforts with government initiatives to screen high-risk regions has greatly increased the use of hepatitis E diagnostic. Other growth drivers include advancements in diagnostic technologies, such as PCR-based tests that help increase accuracy and speed in detecting the virus during its acute phase.
Challenges
- Lack of standardized diagnostic tools: The lack of standardized diagnostic tools for hepatitis E, indeed, represents an important challenge in the accurate diagnosis of the disease. To date, there is no globally accepted test, and it is based on considerable variability in the sensitivity, specificity, and overall performance of available diagnostic assays, which makes it somewhat impractical for interlaboratory or international comparison. This variability, of course, will naturally make cross-regional study of etiology, surveillance, or management of the respective disease challenging. Standardized testing protocols are necessary to ensure reliable and comparable diagnostic outcomes worldwide.
- Cost of advanced diagnostic: The major challenge in the universal application of hepatitis E diagnosis is the cost of the advanced diagnostic tools: PCR and genotyping assays, which consume significantly high resource inputs. These molecular tests are sensitive and specific but require expensive equipment, reagents, and personnel. This diagnostic workup is expensive, making it inaccessible; therefore, early diagnosis and management of hepatitis E remain impossible in most vulnerable populations. Further access to low-cost diagnostic technologies can help advance public health outcomes.
Hepatitis E Diagnostic Tests Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
4.5% |
|
Base Year Market Size (2025) |
USD 66.61 million |
|
Forecast Year Market Size (2035) |
USD 103.44 million |
|
Regional Scope |
|
Hepatitis E Diagnostic Tests Market Segmentation:
Test Type (ELISA HEV IgM Test, ELISA HEV IgG Test, Rapid Diagnostic Test, Polymerase Chain Reaction)
ELISA HEV IgM test segment is set to account for hepatitis E diagnostic tests market share of more than 51.5% by the end of 2035, due to its sensitivity and specificity which could be used in both clinical and epidemiological studies. A closer examination of this assay can help confirm acute hepatitis E and its crucial role in epidemic surveillance. In addition to its relative ease in use and facilitation of handling several samples at one time increases its utility in different healthcare settings it serves as a pivotal diagnostic tool for the effective management of the disease thus contributing to determining the treatment course and fueling the frequency of diagnostic tests.
Sample Type (Blood, Stool)
In hepatitis E diagnostic tests market, blood segment is likely to hold revenue share of more than 85.1% by 2035, owing to the increasing need for proper diagnosis and detection of infection from hepatitis E virus (HEV). The molecular techniques are highly preferred due to the ease and reliability offered by the test results through blood, and this trend is further supported by increasing awareness about the routes of transmission of hepatitis E, such as contaminated water and foodstuffs, which throws light upon the need for proper screening techniques.
Other growth drivers include the rising cases of hepatitis E in endemic areas, along with increased efforts toward healthcare initiatives to control viral hepatitis. Some advanced technologies, such as point-of-care testing and more complex laboratory diagnostic, make blood-based tests more efficient and accessible and thus, hepatitis E diagnostic tests will be boding remarkably for the future growth in the blood segment.
Our in-depth analysis of hepatitis E diagnostic tests market includes the following segments:
|
Test Type |
|
|
Sample Type |
|
|
End user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Hepatitis E Diagnostic Tests Market Regional Analysis:
North America Market Statistics
North America industry is estimated to dominate majority revenue share of 44.6% by 2035, owing to its well-developed health infrastructure, research funding, and robust regulatory frameworks. The growth is essentially propelled by the increasing prevalence of HEV infections, particularly among immunocompromised populations, and rising awareness of the disease, its signs and symptoms among healthcare providers and patients. The major players in the North America diagnostic landscape include Abbott Laboratories and Roche Diagnostic who have developed highly sensitive and specific serological tests and molecular assays facilitating early detection and accurate diagnosis of HEV.
Also, the advent of rapid diagnostic tests has dramatically improved the accessibility of hepatitis E testing in both clinical and field settings with advanced diagnostic tools such as PCR and next-generation sequencing for sensitive detection and differential separation of HEV strains, which is a requirement for conducting epidemiological studies as well as for developing vaccines to fuel the further growth of the hepatitis E diagnostic tests market. Public health programs, combined interagency, academic and private sector collaborations, along with public education have significantly improved the position of the region regarding diagnostic for hepatitis E thus making North America set up the benchmark for the ongoing battle against this infectious disease.
The U.S. hepatitis E diagnostic tests market is relatively niche but growing steadily with increases in awareness and improvements in technologies that are used for diagnosis. Although the disease is more frequently reported in developing countries, sporadic cases and outbreaks in the U.S., coupled with increased international travel, require better diagnostic. The key market players concentrate on enzyme-linked immunosorbent assays and PCR tests that help identify the virus in its acute phase. The market in the U.S. is also driven by the initiatives promulgated by organizations such as the Centers for Disease Control (CDC) which is relentlessly running awareness campaigns to fuel the market growth.
Asia Pacific Market Analysis
Asia Pacific is the fastest-growing hepatitis E diagnostic tests market owing to rapid urbanization, expanding middle-income groups, and technological advancements. The digital transformations, consumer buying capacity, and investments in infrastructure on a large scale have changed the landscape of hepatitis E diagnostic tests. Importantly, collaborations between the companies and government initiatives have been the primary drivers for this growth.
The China hepatitis E diagnostic tests market is driven by the government's focus on strengthening pharmaceutical manufacturing in the country. For instance, China's Made in China 2025 promotes local manufacturing and expertise in technology. Huge corporations like Samsung and Alibaba have started their joint ventures to exploit the region's potential markets in e-commerce, renewable energy, and smart technologies. Besides, policies of local governments encourage investment-friendly circumstances, providing incentives for FDI and friendly conditions of regulatory frameworks. Such a partnership between the private sector and public policies will be crucial for the long-term economic sustainability of the country and will attract global investments.
India is expected to emerge as a key competitor in the global pharmaceutical manufacturing sector during the forecast period. In 2022, the Indian Council of Medical Research began working to improve hepatitis E diagnostic capabilities in the endemic area with the development of quick test kits to aid in early detection. Also, a partnership between pharmaceutical company GSK and the University of Queensland, has been working on developing diagnostic tools as well as vaccines for hepatitis E, working to improve detection methods and response strategies in high-risk populations.
The partnership between the Gates Foundation and diagnostic start-ups has enhanced the point-of-care testing of hepatitis E in low-resource settings, hoping to accelerate timely diagnosis and access to treatment. These partnerships signify an increasing need to pool the resources and expertise of both the public and private sectors to combat hepatitis E effectively.
Key Hepatitis E Diagnostic Tests Market Players:
- Altona Diagnostic GmbH
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- CTK Biotech, Inc.
- Dia.Pro Diagnostic Bioprobes s.r.l
- DiaSorin S.p.A.
- ELITechGroup (Bruker company)
- F. Hoffmann-La Roche Ltd
- Fortress Diagnostic
- Genscript Biotech Corporation
- Guangzhou Wondfo Biotech Co., Ltd.
- Medsource Ozone Biomedicals Pvt. Ltd.
- MIKROGEN GmbH
- MP Biomedicals
- Primer Design
- Wantai BioPharm
Numerous firms have set up initiatives to conduct diagnostic tests for hepatitis E, which is an important public health concern. Companies that deal with hepatitis E diagnostic tests vary with different approaches in conducting the test, which may include serological assays targeting antibodies and molecular tests targeting the identification of the virus's genetic material. Advances in diagnostic technology focus on increasing the efficiency, speed, and accessibility of hepatitis E testing. As awareness about the disease increases, these companies play an important role in promoting early detection and efficient management, further improving healthcare outcomes and surveillance in the infected regions. The prominent players with valuable contributions are:
Recent Developments
- In November 2023, Roche introduced the automated Elecsys Anti-HEV IgM and Elecsys Anti-HEV IgG immunoassays to identify hepatitis E virus (HEV) infections in nations that accept the CE mark, such as the European Union.
- In November 2023, the WHO published the 2023 Essential Diagnostic List (EDL) and added the hepatitis E virus (HEV) to this list along with a recommendation to diagnose acute HEV infections, particularly in immunocompromised patients and pregnant women, using nucleic acid tests (NATs) such as PCR assays.
- Report ID: 6507
- Published Date: Aug 12, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Hepatitis E Diagnostic Tests Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




