Exocrine Pancreatic Insufficiency Treatment Market Outlook:
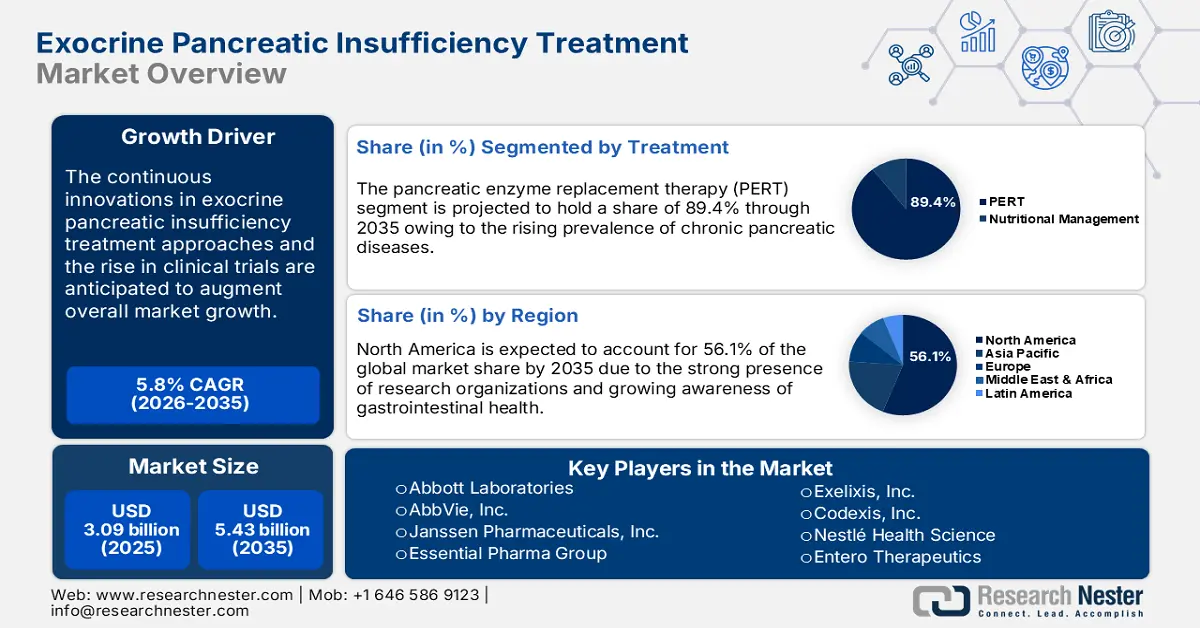
Exocrine Pancreatic Insufficiency Treatment Market size was over USD 3.09 billion in 2025 and is poised to exceed USD 5.43 billion by 2035, growing at over 5.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of exocrine pancreatic insufficiency treatment is estimated at USD 3.25 billion.
The increasing incidence of conditions such as cystic fibrosis, chronic pancreatitis, and pancreatic cancer, which are leading causes of exocrine pancreatic insufficiency (EPI) are anticipated to augment the demand for EPI therapeutic approaches in the coming years. The Cystic Fibrosis Foundation study across 94 countries reveals that there are over 105,000 individuals living with cystic fibrosis. Furthermore, the National Center for Biotechnology Information (NCBI) reveals that chronic pancreatitis leads to 30% to 90% of EPI prevalence rates. The long disease duration and alcoholic etiology are prime factors associated with exocrine pancreatic insufficiency occurrence. The older population who are more susceptible to conditions such as chronic pancreatitis and pancreatic cancer are also the prime contributors to EPI drug sales.
The expansion of immunology and gut health product offerings is aiding the market players in uplifting their revenue shares. For instance, in October 2024, AbbVie, Inc. revealed its third quarter 2024 financial results. As per the report, the company totaled global net revenues of 14.46 billion with a rise of 3.8% on a reported basis and 4.9% on an operational basis. The worldwide immunology portfolio's net revenues were evaluated at USD 7.04 billion an increase of 4.8% on an operation basis and 3.9% on a reported basis. Furthermore, the R&D expense on the generally accepted accounting principle (GAAP) basis was 14.7% of net revenues.