Enzyme Replacement Therapy Market Outlook:
Enzyme Replacement Therapy Market size was valued at USD 12.74 billion in 2025 and is likely to cross USD 29.61 billion by 2035, registering more than 8.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of enzyme replacement therapy is assessed at USD 13.75 billion.
The growth of the market can be attributed to rising prevalence of lysosomal storage diseases (LSDs) in the population owing to rising diseases and an unhealthy lifestyle. There were 766 LSD diagnoses in the Australian population during the 12-year period, January 2009 through December 2020, including 32 successful pregnancies.
Also, the growing population is expected to boost the market further over the forecast period. The growth in population is anticipated to lead a rise in the prevalence of rare diseases, which is also expected to fuel the demand for enzyme replacement therapy. The population of the world is projected to rise by 2 billion people during the next 30 years, from 7.7 billion people today to 9.7 billion in 2050, with a potential peak of approximately 11 billion people around the year 2100.
The worldwide incidence of lysosomal storage illnesses, including Gaucher, Fabry, Pompe, and MPS, is steadily rising. For instance, as per information released by the National Institute of Neurological Disorders and Stroke in August 2021, approximately one in 40,000 individuals in the United States had Pompe disease, which is predicted to be roughly 32,950 individuals. Furthermore, the National Fabry Foundation estimates that the entire Fabry patient number in the USA will be roughly 7,713 in May 2020. As a result of the significant number of patients experiencing rare lysosomal storage illnesses, there is a huge requirement for efficient treatments, including enzyme replacement therapy.
Key Enzyme Replacement Therapy Market Insights Summary:
Regional Highlights:
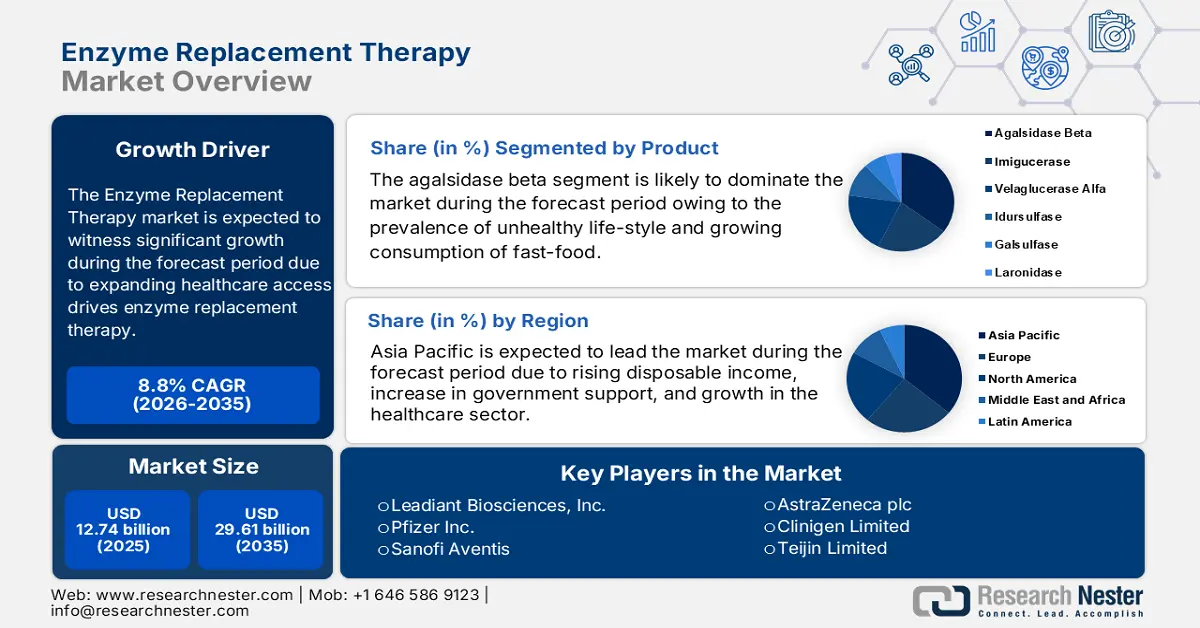
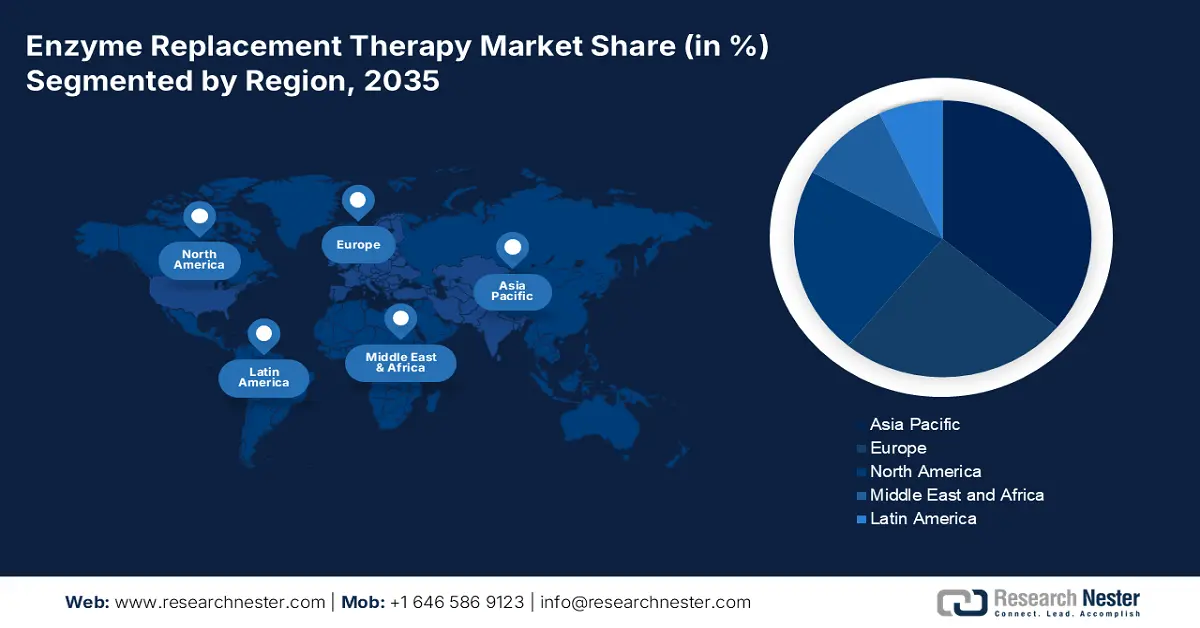
- The Asia Pacific enzyme replacement therapy market commands the largest share by 2035, driven by rising patient population, disposable income, healthcare growth, and government support.
- The Europe market will secure the second largest share by 2035, attributed to supportive regulatory policies and advancements in medical infrastructure.
Segment Insights:
- The agalsidase beta segment in the enzyme replacement therapy market is anticipated to capture the largest share by 2035, fueled by chronic disease prevalence and rising adoption for Fabry disease treatment.
- The exocrine pancreatic insufficiency segment in the enzyme replacement therapy market will command the largest share, propelled by the high incidence of EPI in chronic pancreatitis and cystic fibrosis patients, forecast year 2035.
Key Growth Trends:
- Growing Government Spending on Healthcare Infrastructure
- Rise in Prevalence of Rare Disease
Major Challenges:
- Growing Government Spending on Healthcare Infrastructure
- Rise in Prevalence of Rare Disease
Key Players: BioMarin, Leadiant Biosciences, Inc., Pfizer Inc., Sanofi Aventis, AbbVie Inc., Takeda Pharmaceutical Company Limited, JCR Pharmaceutical Co., Ltd., AstraZeneca plc, Clinigen Limited, Teijin Limited.
Global Enzyme Replacement Therapy Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 12.74 billion
- 2026 Market Size: USD 13.75 billion
- Projected Market Size: USD 29.61 billion by 2035
- Growth Forecasts: 8.8% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: Asia Pacific
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, China, Japan, Germany, France
- Emerging Countries: China, India, Japan, South Korea, Brazil
Last updated on : 9 September, 2025
Enzyme Replacement Therapy Market Growth Drivers and Challenges:
Growth Drivers
- Growing Government Spending on Healthcare Infrastructure – Governments of different nations are focusing on expanding the healthcare sector to treat people and combating health issues. As a result of rising investment in healthcare infrastructure, the size of enzyme replacement therapy market is expected to expand in the assessment period. According to an estimate, in 2020, the United States spent more than USD 3 trillion on healthcare.
- Rise in Prevalence of Rare Disease - There are between 1,000 and 2,000 new occurrences of ALSP each year, which is a rare, inherited, autosomal dominant neurological condition with strong penetrance that affects an estimated 10,000 persons in the US.
- Growing Cases of Pompe Disease - Since enzyme replacement therapy (ERT) serves as the only viable treatment option for Pompe disease. Therefore, increasing cases of the illness are anticipated to contribute to market expansion. ERT has contributed to disease course stabilization with motor and pulmonary modifications in patient populations with late-onset Pompe disease. According to experts, there are between approximate 4,000 and about 9,000 cases of Pompe disease globally.
- Technology Advancement - The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended that Lamzede (velmanase alfa), a long-term enzyme replacement therapy for adults, adolescents, and children with mild to moderate forms of alpha-mannosidosis, be given marketing authorization in the European Union (EU).
- Growing Genetic Disorder - A genetically based ailment will affect roughly 6 out of every 10 individuals across the globe.
Challenges
- Lack of Awareness - Lack of awareness about enzyme replacement therapy among developing nations is slowing down the adoption rate of enzyme replacement therapy. Also, the deficiency of technology and adequate infrastructure is expected to hamper the market growth.
- High Cost of Enzyme Replacement Therapy
- Stringent Reimbursement Policies
Enzyme Replacement Therapy Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
8.8% |
|
Base Year Market Size (2025) |
USD 12.74 billion |
|
Forecast Year Market Size (2035) |
USD 29.61 billion |
|
Regional Scope |
|
Enzyme Replacement Therapy Market Segmentation:
Product Segment Analysis
The agalsidase beta segment is anticipated to garner the largest revenue by the end of 2035, backed by increasing prevalence of chronic disease in the population across the world owing to consumption of unhealthy food items and an engaged damaging lifestyle. Between 2000 and 2030, it was predicted that the number of Americans with chronic diseases would increase by around 45 million, or roughly 36%. Furthermore, the agalsidase segment held the second-largest market share in 2023. The increased cost and growing adoption of this medication in the treatment of Fabry disease are the principal factors for this segment's substantial market share.
Disease Segment Analysis
The exocrine pancreatic insufficiency segment will capture the largest enzyme replacement therapy market share by 2035, owing to the increasing incidence of this ailment amongst the general population. Furthermore, the high pervasiveness of exocrine pancreatic insufficiency among individuals suffering from chronic pancreatitis and cystic fibrosis contributes to a rise in the disease's patient group. This, in turn, contributes to this segment's supremacy over the forecast period. According to a Cleveland Clinic organization report, nearly 9 out of 10 infants with cystic fibrosis develop EPI during the first year. The remainder is in danger of developing EPI as children or adults.
Our in-depth analysis of the global enzyme replacement therapy market includes the following segments:
|
By Product |
|
|
By Disease |
|
|
By End-User |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Enzyme Replacement Therapy Market Regional Analysis:
Asia Pacific Market Insights
The Asia Pacific enzyme replacement therapy market, amongst the market in all the other regions, is projected to hold the largest market share by the end of 2035, owing to the growing number of patient population suffering from rare disorders, rising disposable income, growth in the healthcare sector, and an increase in government support. According to the Economist Intelligence Unit, 258 million individuals in Asia and the Pacific region suffer from rare diseases.
Europe Market Insights
Europe will hold second-largest share of the worldwide market. The increase can be credited primarily to supportive regulatory policies in a few European nations for different infrequent lysosomal ailments, including Gaucher disease, MPS, and others. As per F1000 Research Ltd., the Polish National Health Fund, for instance, implemented a national drug program in 2019, with the first remuneration in ERT for Fabry disease. Furthermore, rapid advancements in medical infrastructure and a growing preference for therapeutic approaches for rare diseases drive market expansion in this region.
Enzyme Replacement Therapy Market Players:
- BioMarin
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Leadiant Biosciences, Inc.
- Pfizer Inc.
- Sanofi Aventis
- AbbVie Inc.
- Takeda Pharmaceutical Company Limited
- JCR Pharmaceutical Co., Ltd.
- AstraZeneca plc
- Clinigen Limited
- Teijin Limited
Recent Developments
-
Teijin Limited announced the immediate availability of Revcovi 2.4 mg for intramuscular injection [Elapegademase (Genetic Recombination)]. With Revcovi 2.4 mg, Japan's first medication for Adenosine Deaminase (ADA) Deficiency, individuals with ADA Deficiency are expected to have improved ADA activity and immune system.
-
Clinigen Limited, a global pharmaceutical Products, and Services company, announced that Clinigen K.K., a fully owned subsidiary of Clinigen located in Tokyo, has gained manufacturing and marketing authorization for Hunterase (Idursulfase-beta) ICV 15mg as part of its strategic relationship with GC Pharma. The clearance of Hunterase ICV in Japan marks its first appearance in any nation globally.
- Report ID: 4455
- Published Date: Sep 09, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Enzyme Replacement Therapy Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




