Clinical Disorder Treatment Market - Regional Analysis
North America Market Insights
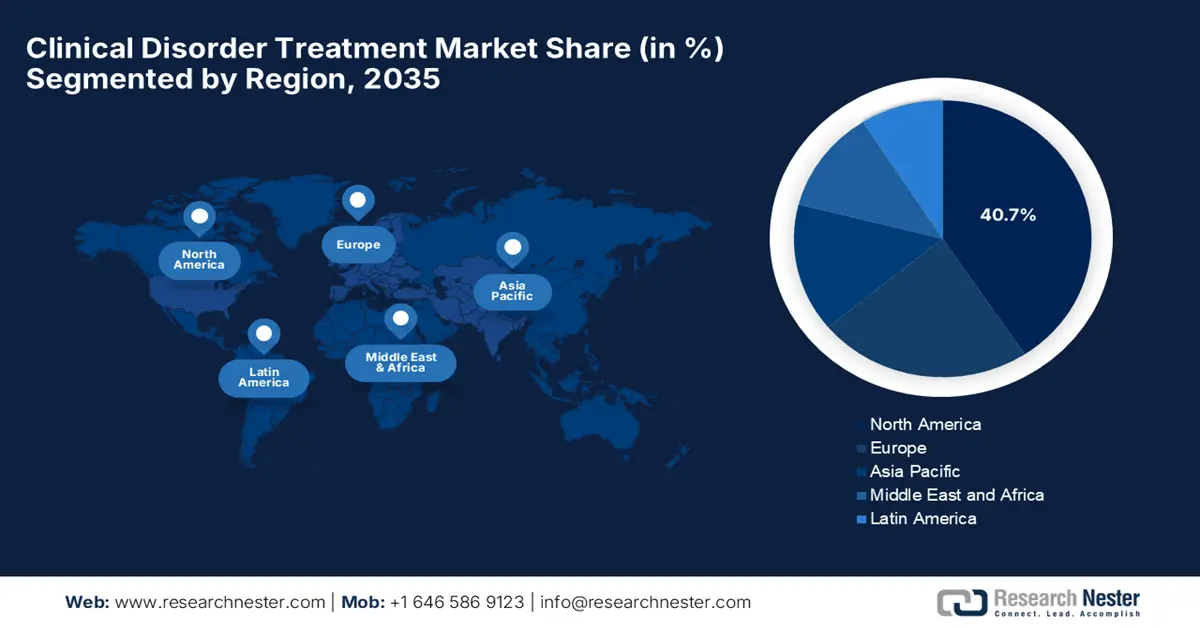
North America is anticipated to hold a significant position in the global clinical disorder treatment market by capturing the largest revenue share of 40.7% by the end of 2035. The region’s growth in this sector is effectively attributable to the increasing funding grants and the presence of advanced healthcare infrastructure. The Biden-Harris administration in September 2024 announced a major investment of USD 68.5 million to address the U.S. mental health crisis, with a prime focus on expanding the workforce and improving care for underserved communities. Besides, USD 2 million was allocated through the LEAP in Health IT program to enhance interoperable medical systems that improve patient data access and care coordination.
The U.S. is the key contributor to growth in the clinical disorder treatment market, effectively propelled by the presence of payers, manufacturers, and policymakers who increasingly prioritize mental health and neurological care. In January 2025, Johnson & Johnson reported that the U.S. FDA had accepted its SPRAVATO (esketamine) nasal spray, which is the first and only monotherapy for adults with treatment-resistant depression who have not responded to at least two oral antidepressants, hence denoting a positive market outlook.
In Canada, the market is expected to garner humongous growth due to significant investment from the provincial and federal governments. In September 2025, the Government of Canada reported that it is investing over USD 30 million over four years to expand the Integrated Youth Services Network to improve mental health services for youth aged 12 to 25. The initiative was successfully led by CIHR and Indigenous Services Canada, supports 12 regional networks and an Indigenous network to enhance research and data systems, and is hence suitable for standard market growth.
Key Financial Burdens Across Mental and Neurological Disorders
|
Disorder / Area |
Estimated Cost Impact |
|
Alzheimer’s Disease |
USD 360 billion (2024); projected USD 1 trillion by 2050 |
|
Epilepsy |
USD 13.4 billion total (2019); USD 5.4 billion directly attributable |
|
Schizophrenia |
USD 30,000-USD 60,000 per patient annually (estimates from external sources) |
|
Depression |
Patients incur 2.5× higher healthcare costs (indirect estimate) |
Source: CDC
APAC Market Insights
Asia Pacific is identified as the fastest-growing region in the clinical disorder treatment market over the analyzed timeframe. The region’s progress in this field is highly subject to the rising recognition of mental health challenges alongside growing non-communicable disease burdens. On the other hand, the increasing urbanization, shifting lifestyles, and other social factors have created a huge demand for depression, anxiety, and mood disorder therapies. Furthermore, the governments across the region are extensively supporting telehealth and digital health platforms and improving regulatory pathways for psychiatric drugs.
China has a strong potential in the regional clinical disorder treatment market backed by policy reforms, increased public awareness, and technological adoption. The country also benefits from expanding outpatient services and regional mental health specialty centers that effectively help manage these conditions. For instance, in June 2023, Brii Biosciences reported the dosing of the first subject in a Phase 1 clinical trial of BRII-297, which is a novel long-acting injectable designed for the treatment of anxiety and depressive disorders. Moreover, BRII-297 is a GABAA receptor positive allosteric modulator and represents a first-of-its-kind approach.
India in the clinical disorder treatment market is portraying steady growth owing to the heightened demand for treatments for depression, anxiety, PTSD, and autism, supported by growing public and private investments. The Ministry of Finance in July 2024 reported that, through the Economic Survey 2023 - 2024, it found that 10.6% of adults in the country suffer from mental disorders, wherein treatment gaps range from 70% to 92%. The Survey also noted that over 8.07 lakh calls had been handled under the Tele MANAS programme, which is supported by 53 cells across 34 States/UTs.
Europe Market Insights
The clinical disorder treatment market in Europe is predicted to register a considerable growth rate during 2026-2035, fueled by a surge in novel product introductions and a rising aging population in the region. In August 2025, Eisai Co., Ltd. and Biogen Inc. announced that they had officially launched LEQEMBI in Austria, with a subsequent launch scheduled for Germany. The product is approved by the European Commission and is the first treatment in the region targeting the underlying cause of Alzheimer’s disease, specifically indicated for patients with early AD who are ApoE ε4 non-carriers or heterozygotes with confirmed amyloid pathology.
In Germany, the clinical disorder treatment market is experiencing significant growth on the back of the introduction of policy reforms and digital integration. In March 2024, Boehringer Ingelheim reported that it joined forces with Sosei Heptares to develop GPR52 agonists for the treatment of schizophrenia. Further, the prime focus of this collaboration revolves around HTL0048149, which is a first-in-class GPR52 agonist currently in Phase 1 trials, and has the potential to become a precision medicine targeting the brain regions involved in schizophrenia.
The U.K. is also maintaining a strong position in the clinical disorder treatment market since the public health innovations are prioritizing digital and therapeutic tools, integrating virtual reality and telehealth to reach people more efficiently. For instance, BDD Pharma in April 2025 announced that its OralogiK oral delivery technology is driving the development of CTx‑2103, which is a once-daily formulation of buspirone aimed at treating anxiety disorders. Hence, this highlights the growing role of advanced formulation platforms in making psychiatric therapies extremely convenient.