Chlamydia Infection Diagnostics Market Outlook:
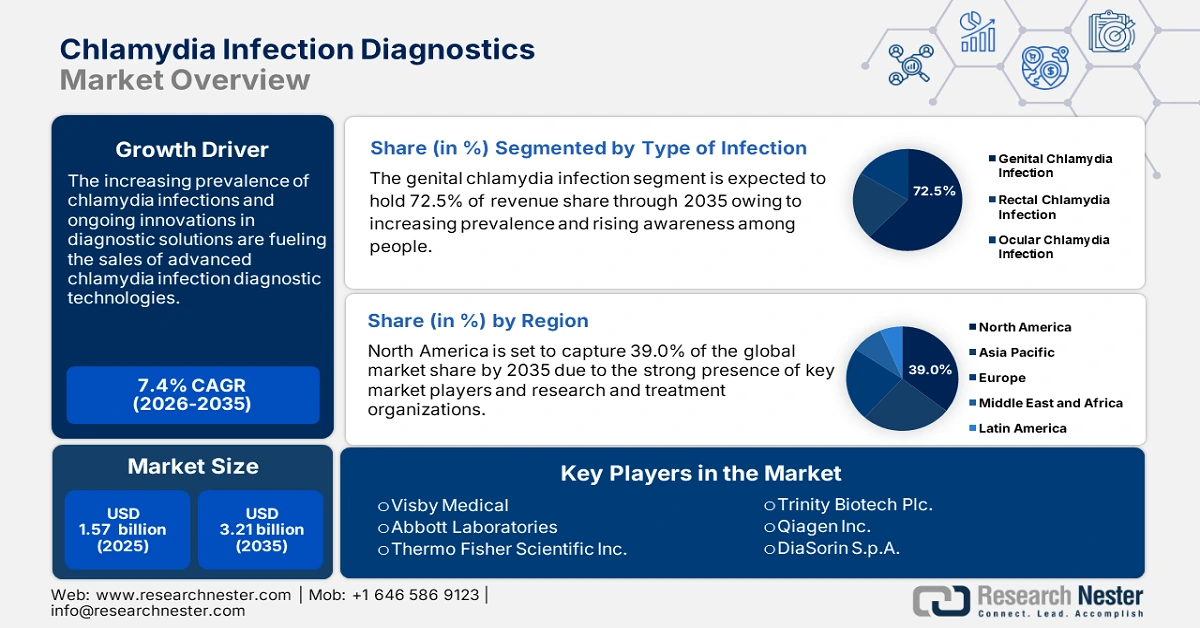
Chlamydia Infection Diagnostics Market size was over USD 1.57 billion in 2025 and is projected to reach USD 3.21 billion by 2035, growing at around 7.4% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of chlamydia infection diagnostics is evaluated at USD 1.67 billion.
Chlamydia is asymptomatic and many young individuals are unaware of STDs and associated risks. This lack of awareness contributes to its higher transmission rates, fuelling the need for advanced chlamydia infection diagnostic solutions. As the prevalence rate increases, the need for effective diagnostic solutions to identify and treat infections promptly booms. This demand drives innovation and investment in chlamydia testing technologies.
For instance, according to the World Health Organization (WHO), around 128.5 million new chlamydia infection cases were registered among the age group of 15 to 49 across the world, in 2022. Particularly for this demographic, the prevalence rate among women was 4.0% and 2.5% in men. Untreated chlamydia can cause serious issues such as HIV or other STIs. To minimize the prevalence rate of chlamydia, public and private healthcare organizations are implementing targeted screening programs and educational campaigns, further boosting the sales of diagnostic solutions.
Key Chlamydia Infection Diagnostics Market Insights Summary:
Regional Highlights:
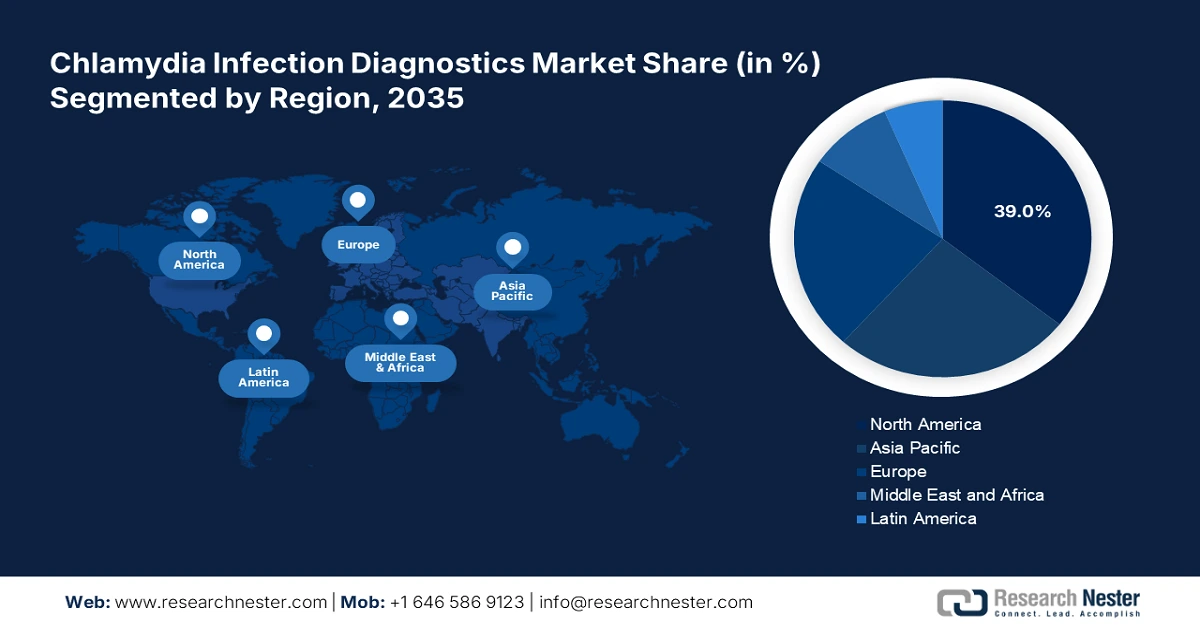
- North America leads the Chlamydia Infection Diagnostics Market with a 39% share, supported by advanced research organizations and high STI screening initiatives, driving growth through 2026–2035.
- Rapid growth is anticipated in Asia Pacific's chlamydia infection diagnostics market by 2035, attributed to increasing healthcare expenditures and rising awareness of STIs.
Segment Insights:
- The Hospitals segment is expected to maintain a 52.5% market share by 2035, propelled by the availability of integrated healthcare services and diagnostics.
- Genital Chlamydia Infection segment is expected to capture a 72.50% share by 2035, driven by high prevalence and advancements in diagnostic tools like NAATs.
Key Growth Trends:
- Advancements in POCT
- At-home testing kits gaining traction
Major Challenges:
- Stigma and cultural influences
- Lack of knowledge
- Key Players: Visby Medical, Abbott Laboratories, Thermo Fisher Scientific Inc., Trinity Biotech Plc., Qiagen Inc., and Becton, Dickinson and Company.
Global Chlamydia Infection Diagnostics Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.57 billion
- 2026 Market Size: USD 1.67 billion
- Projected Market Size: USD 3.21 billion by 2035
- Growth Forecasts: 7.4% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (39% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, United Kingdom, France, Japan
- Emerging Countries: China, India, Brazil, Mexico, Singapore
Last updated on : 14 August, 2025
Chlamydia Infection Diagnostics Market Growth Drivers and Challenges:
Growth Drivers
- Advancements in POCT: Innovations in point-of-care testing (POCT), rapid tests, and molecular diagnostic techniques are enhancing the accuracy and speed of chlamydia detection. Point-of-care testing is medical diagnostic testing performed at or near the site of the patient, which offers several advantages including rapid results, convenience, and the potential to increase screening rates. Molecular diagnostics particularly polymerase chain reaction have revolutionized chlamydia detection by enabling the identification of genetic material from chlamydia trachomatis with high sensitivity and specificity. Advanced molecular techniques can distinguish between different strains of chlamydia, aiding in epidemiological studies and targeted treatment, driving their demand growth.
- At-home testing kits gaining traction: The emergence of user-friendly home testing kits is anticipated to represent a significant shift in healthcare accessibility in the coming years. Chlamydia home testing kits allow patients to conduct tests at their convenience, eliminating the need for clinic visits, which can be inconvenient for some. Modern home testing kits also offer online portals or apps for result tracking, providing a comprehensive approach to health management. Furthermore, these kits have the potential to increase diagnostic rates leading to better public health outcomes. For instance, in November 2023, the U.S. Food and Drug Administration granted the commercialization of LetsGetChecked for the Simple 2 Test. This is the first at-home diagnostic test for chlamydia and gonorrhea and is easily available over the counter for adult patients over 18 years of age.
Challenges
- Stigma and cultural influences: The stigma associated with sexually transmitted infections including chlamydia can deter people from seeking diagnosis and treatment, affecting overall testing rates. The majority of individuals fear being judged by society if they look for testing or treatment for STIs. This fear can lead to avoidance of healthcare, resulting in missed opportunities for diagnosis.
- Lack of knowledge: Lack of information related to chlamydia and the importance of regular screening is expected to lower the demand for diagnostic testing solutions. Many individuals are unaware of the risk factors associated with chlamydia, which leads to complacency regarding their sexual health. Chlamydia often presents with mild or no symptoms, particularly in women, which creates a belief that they are at no risk resulting in reduced motivation to seek screening.
Chlamydia Infection Diagnostics Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
7.4% |
|
Base Year Market Size (2025) |
USD 1.57 billion |
|
Forecast Year Market Size (2035) |
USD 3.21 billion |
|
Regional Scope |
|
Chlamydia Infection Diagnostics Market Segmentation:
Type of Infection (Genital Chlamydia Infection, Rectal Chlamydia Infection, Ocular Chlamydia Infection)
The genital chlamydia infection segment in the chlamydia infection diagnostics market is expected to capture 72.5% of the revenue share by 2035. Genital chlamydia infection occurs due to the direct transmission of agents from person-to-person urogenital tract to the eyes and vice versa occurs via contaminated fingers, towels, or other fomites. The rising prevalence of this infection among adults and adolescents is heightening the need for effective diagnostic tools. The advancements in diagnostic technologies such as nucleic acid amplification tests (NAATs), offer higher sensitivity and specificity making chlamydia testing more reliable.
End use (Hospitals, Speciality Clinics, Diagnostic Centers)
The hospital segment is foreseen to account for 52.5% of the global chlamydia infection diagnostics market share through 2035 owing to the large patient base and first preference of patients for medical care. Hospitals provide a multidisciplinary approach to healthcare. Patients often prefer them for chlamydia testing because they can access various services, including counseling, treatment, and follow-up care, all in one place. The presence of skilled healthcare professionals and staff, who can offer more informed and reliable diagnostic and treatment options coupled with the presence of next-gen diagnostic technologies, which enable more accurate and faster testing for chlamydia are contributing to the segmental growth.
Our in-depth analysis of the chlamydia infection diagnostics market includes the following segments:
|
Test Type
|
|
|
Type of Infection |
|
|
End use |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Chlamydia Infection Diagnostics Market Regional Analysis:
North America Market Forecast
North America industry is predicted to hold largest revenue share of 39% by 2035, owing to the presence of advanced research organizations and next-gen healthcare facilities. The chlamydia awareness programs and high screening initiatives are also set to fuel the sales of diagnostic solutions and services in the region.
The U.S. chlamydia infection diagnostics market is estimated to reach a valuation of USD 1.5 billion by 2035. The high prevalence of sexually transmitted infections is one of the major drivers pushing the demand for advanced chlamydia infection diagnostic solutions. For instance, according to the Centers for Disease Control and Prevention, in 2022, over 2.5 million cases of gonorrhea, syphilis, and chlamydia were reported in the U.S. These alarming statistics are driving high investments in research and development activities to develop innovative diagnostic and treatment solutions.
Canada is also witnessing rising cases of sexually transmitted infections including chlamydia, promoting a high need for effective diagnostic solutions. The public health organizations in the country are also increasingly focusing on STI prevention, leading to high awareness and demand for testing services. For instance, the Government of Canada has developed an action plan 2024-2030 to mitigate the prevalence of sexually transmitted and blood-borne infections.
Asia Pacific Market Statistics
The Asia Pacific market is anticipated to increase at a rapid pace during the projected period owing to the increasing healthcare expenditures, growing awareness of STIs, and rising entrance of international diagnostic solution manufacturing companies. China and India are the most lucrative marketplaces for key players owing to cost-effective R&D and high investments in healthcare infrastructure development projects. The presence of modern medical facilities and innovative solutions is also driving market growth in Japan and South Korea.
In India, the increasing prevalence rate of STIs including chlamydia is augmenting a high demand for advanced diagnostic solutions. The expanding medical devices and diagnostic equipment markets are also positively influencing the profits of chlamydia infection diagnostic solution manufacturers.
Key Chlamydia Infection Diagnostics Market Players:
- Visby Medical
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Abbott Laboratories
- Thermo Fisher Scientific Inc.
- Trinity Biotech Plc.
- Qiagen Inc.
- ELITechGroup
- Becton, Dickinson and Company
- Bio-Rad Laboratories, Inc.
- Hologic, Inc.
- QuidelOrtho Corporation.
- Siemens Healthineers
- DiaSorin S.p.A.
- F. Hoffmann-La Roche Ltd
Key players in the chlamydia infection diagnostics market employ several tactics such as new product launches, technological advancements, partnerships and collaborations, regional expansion, and more to earn high profits. Industry giants are investing heavily in research and development activities to introduce innovative diagnostic solutions. Strategic collaborations with other players and research organizations are also aiding them to expand their product folios. Furthermore, regional expansion strategies are helping them to tap into emerging markets to cater to a larger audience base.
Some of the key players include:
Recent Developments
- In May 2022, Abbot Laboratories received an FDA clearance for its Alinity m STI Assay solution. It is a first-of-its-kind multiplex test that aids in addressing rising STI rates.
- In August 2021, Visby Medical received a 510(k) clearance and waiver under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) from the US Food and Drug Administration to commercialize its quick, single-use polymerase chain reaction (PCR) diagnostic test. This product is vital for multiplexed detection of sexually transmitted infections including chlamydia trachomatis, using a self-collected vaginal swab.
- Report ID: 6570
- Published Date: Aug 14, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




