Cell & Gene Therapy Manufacturing Services Market Regional Analysis:
North America Market Analysis
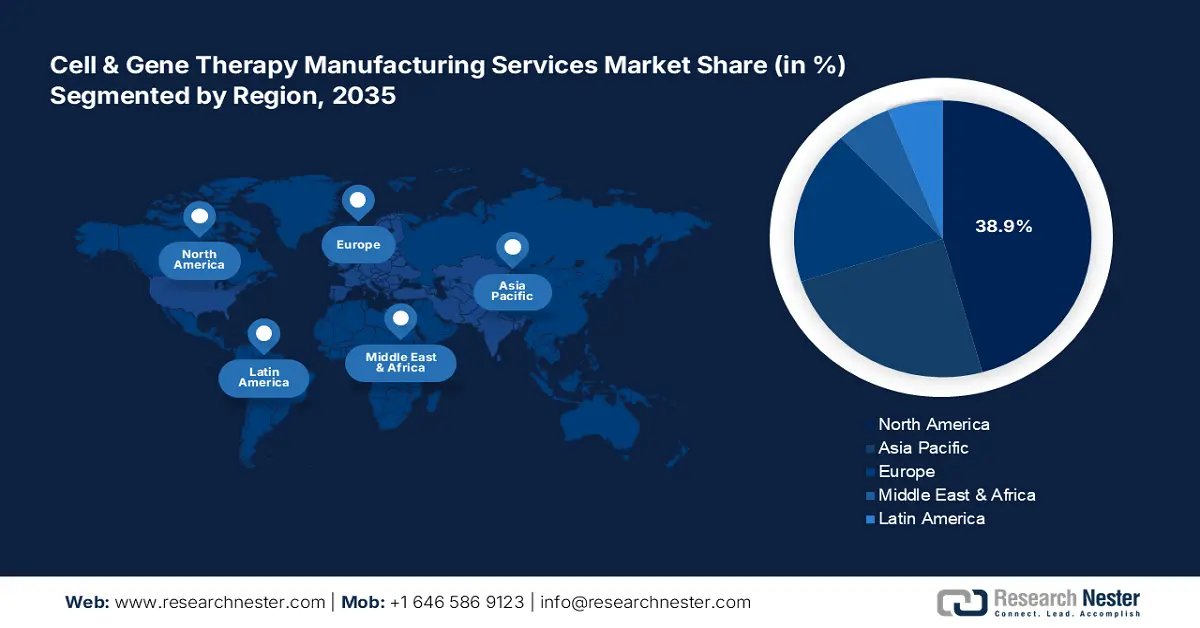
North America industry is anticipated to account for largest revenue share of 38.9% by 2035, Domestic companies in North America have amplified their manufacturing operations in the cell & gene therapy manufacturing services market. Moreover, a strong network of CDMOs enables scalable production of the therapies in the region. In March 2023, Thermo Fisher Scientific Inc., in collaboration with the University of California, San Francisco (UCSF) announced the acceleration of advanced cell therapies for difficult-to-treat conditions. This includes cancer, rare diseases, and other illnesses.
As per the National Cancer Institute, in 2024, an estimated 2,001,140 new cases of cancer were diagnosed in the U.S., leading to nearly 611,720 people dying from the disease. The country dominates the North America region, driven by a mixture of strong technological infrastructure, substantial funding in biotechnology, and an exceedingly skilled workforce. Moreover, supportive regulations have enhanced R&D activities, leading to a flow in the number of clinical trials and approvals of advanced groundbreaking therapies in the market.
Canada market is growing steadily, supported by the government, a strong academic research environment, and collaborative industry partnerships. The country’s focus on innovation and regulatory advancements, such as the Pathway to Licensing advanced therapies is strengthening the cell & gene therapy manufacturing services market in the country. Scaling domestic production is enhancing the supply chain in the country, and accelerating cross-border partnerships.
APAC Market Statistics
APAC region houses a large and assorted population, including a substantial patient number from diseases which can be treated potentially through cell and gene therapies. This grants a considerable market prospect for establishments providing manufacturing services in the region, further driving market growth. The region is also progressively identifying the capabilities of the life sciences segment. These factors are boosting the region’s market.
South Korea market is particularly supported by a robust biotech industry. Regulatory reforms and financial support for biopharmaceuticals have spurred the establishment of cutting-edge production facilities, aiming to position South Korea as a regional hub for cell and gene therapy. For instance, in October 2024, Samsung Biologics launched a new high-concentration formulation platform, S-HiCon, to boost high-dose biopharmaceutical development and manufacturing.
China cell & gene therapy manufacturing services market is driven by a large patient base with unmet medical needs. The government’s commitment to supporting biotech innovation, combined with favorable regulatory adjustments for faster approvals, has fostered an environment where biotech companies can rapidly advance cell and gene therapies from research to commercialization. Hence, the country’s market is anticipated to witness considerable growth during the forecast period.