CAR T-Cell Therapy Market Outlook:
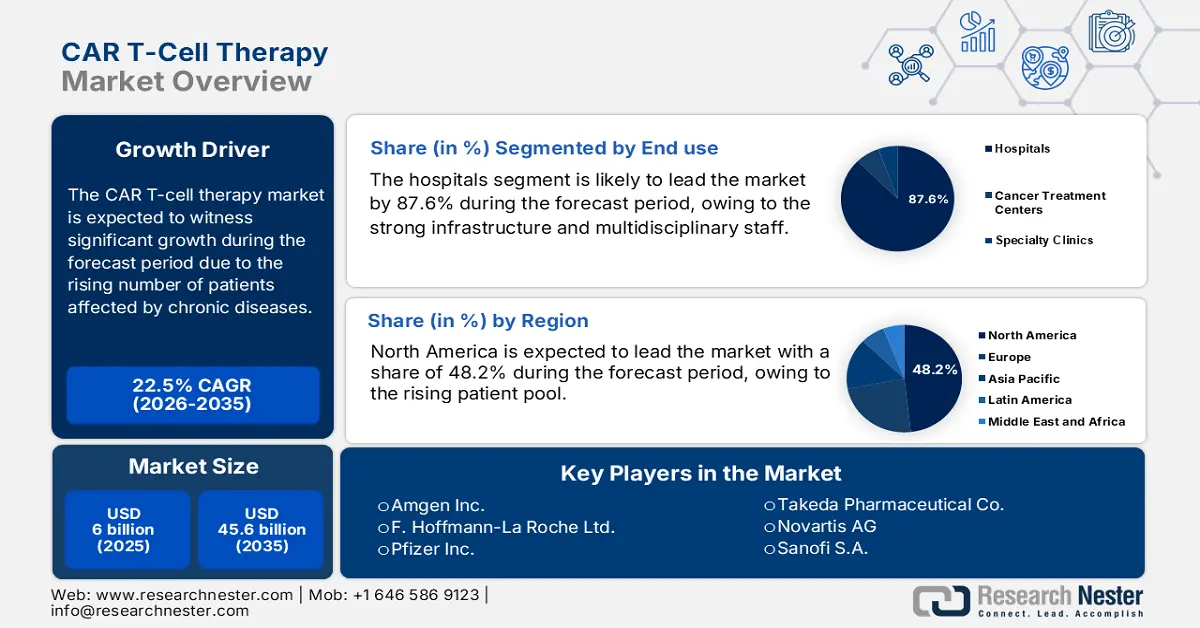
CAR T-Cell Therapy Market size was valued at USD 6 billion in 2025 and is projected to reach USD 45.6 billion by the end of 2035, rising at a CAGR of 22.5% during the forecast period, i.e., 2026-2035. In 2026, the industry size of CAR T-cell therapy is assessed at USD 7.35.
The market is driven by the rising number of patients affected by chronic diseases, and the demand for targeted treatment also rises, creating a sustainable consumer base for the market. According to the NLM study published in May 2025, nearly 1,580 CAR T clinical trials were registered at ClinicalTrials.gov as of April 2024. Public funders and nonprofits remain large sponsors of trials and translational studies; NIH and disease foundations provide core grant funding and trial support that underpin clinical growth and broaden the addressable pool. This demographic trend represents a landscape of biopharmaceutical advances, benefiting this sector.
CAR T cells are mostly used to modify the genetic cytotoxic immune T cells to approach tumor-specific antigens and support durable remissions of relapsed or refractory B-Cell Lymphoma. B-Cell lymphoma is the most prevalent form of malignant lymphoma, and relapsed or refractory lymphoma has been demonstrated to be a greater cause of treatment failure. For example, according to Cancer Network, June 2022, diffuse large B-cell lymphoma (DLBCL) maintains a considerable presence under the category of Non-Hodgkin Lymphoma, and about 30-40% of the patients develop relapsed/refractory DLBCL in the first 2 years. Moreover, different product launches and approvals impart substantial momentum to the industry players. Various shortages and volatilities in manufacturing plants and API production are recognized as the key drivers of this upstream price flow.