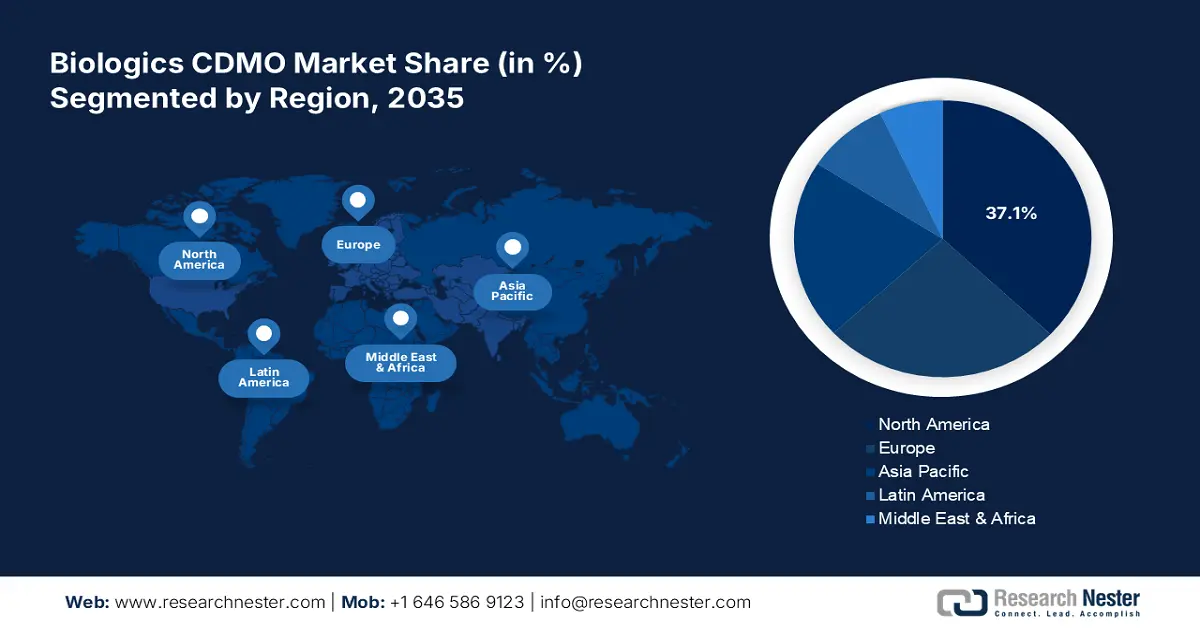
Biologics CDMO Market Regional Analysis:
North America Market Insights
North America in biologics CDMO market is set to capture over 37.1% revenue share by 2035. North America is at the forefront of developing cutting-edge therapies, including gene editing and cell therapies, which drives the demand for customized information capabilities. As these therapies continue to grow, pharmaceutical companies increasingly outsource processing by fostering strategic partnerships. These collaborations enhance efficiency, streamline engineering processes, and reduce costs, contributing significantly to the expansion of the biological creation sector. The combination of innovation and outsourcing creates a dynamic growth environment for biological fabrication in North America.
The increasing prevalence of chronic diseases, cancer, and autoimmune disorders in the U.S. is driving the demand for recombinant products, which necessitates efficient fabrication solutions provided by CDOs. To meet this growing demand, U.S. companies are investing heavily in fabrication infrastructure to extend their formation capacity. The President's pledge to expand the bioeconomy prompted USD 46.0 billion in investments in bioprocessing projects in the public and commercial sectors, according to a White House report released in November 2024. These investments enable them to scale production, improving efficiency and supporting large-scale processing. Such growing investments drive U.S. biologics CDMO market expansion.
The shift toward personalized medicine in Canada, including targeted therapies and gene-based treatments, is driving the demand for genetically altered products, which requires specialized creation handled by CROs. To meet this growing demand, Canada is heavily investing in expanding its bio formulation infrastructure, including new facilities and the expansion of existing ones. In July 2021, the government of Canada stated that it spent more than USD 1.2 billion to rebuild the country's capacity for vaccines, medicines, and biomanufacturing. This investment enabled CDOs to scale up processing, offer comprehensive services, and meet global demand, significantly contributing to the growth of the biologics CDMO market in Canada.
APAC Market Insights
In APAC, the biologics CDMO market is set to garner the fastest CAGR over the forecast period. The rapid expansion of the biopharmaceutical industry in this region, driven by increasing healthcare needs and advancements in biotechnology, is boosting the demand for genetically modified products. With patent expirations for blockbuster biologics, the demand for biosimilars has surged. CROs play a crucial role in supporting the formulation of both biologics and biosimilars, driving market growth by providing specialized formation capabilities. Their involvement in the development and formation of these therapies contributes significantly to the expansion of the biologics CDMO market in the region.
China is becoming a global hub for R&D, with numerous pharmaceutical and biotech companies focusing on biologic drug development. This growing number of biological candidates drives demand for specialized creation services. Additionally, the expanding economy and improving healthcare infrastructure in China coupled with increasing disposable income and better healthcare access, contribute to the rising demand for advanced biologic therapies. These factors collectively fuel the growth of the biologics CDMO market, positioning China as a key player.
The growing biotechnology sector in India is fueled by both public and private investments, driving demand for drug development, including vaccines, monoclonal antibodies, and biosimilars. This demand boosts the need for specialized outsourced services. Additionally, the government of India, favorable policies, such as the National Biopharma Mission, support the pharmaceutical and biotechnology industries, creating a favorable environment for bio-synthetic production. For instance, in IBEF November 2024, India's biotechnology sector doubled to 70.2 billion in 2020 and is expected to reach USD 150 billion in 2025. These factors collectively contribute to the acceleration of the biologics CDMO market in India.