Bilirubin Blood Test Market Outlook:
Bilirubin Blood Test Market size was valued at USD 1.57 billion in 2025 and is likely to cross USD 2.87 billion by 2035, registering more than 6.2% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of bilirubin blood test is assessed at USD 1.66 billion.

The rising prevalence of liver disorders, such as hepatitis, cirrhosis, and fatty liver disease, as well as neonatal jaundice due to immature liver function, is a growing concern. According to the NIH in August 2023, liver disease accounts for approximately 2 billion deaths annually, representing about 4% of all deaths worldwide (or one out of every 25 deaths). This situation is driving up the demand for bilirubin blood tests. These tests are essential for diagnosing and monitoring liver function in adults and for the early detection and treatment of jaundice in newborns. Increased awareness of liver health and neonatal care is further promoting the adoption of bilirubin testing in healthcare settings around the globe.
The development of portable, point-of-care (POC), and non-invasive bilirubin testing devices has significantly transformed the diagnostic landscape, making bilirubin testing more accessible, efficient, and patient-friendly. According to the National Library of Medicine (NLM) in March 2023, POC devices can provide bilirubin test results up to approximately 60 times faster than traditional laboratory-based quantification. These advanced devices eliminate the need for complex laboratory setups and reduce patient discomfort by enabling rapid and accurate testing at the bedside or in outpatient settings. Their ease of use and reliability have led to increased adoption across various healthcare facilities, including hospitals, clinics, and even home care, ultimately improving overall diagnostic efficiency and patient outcomes.
Key Bilirubin Blood Test Market Insights Summary:
Regional Highlights:
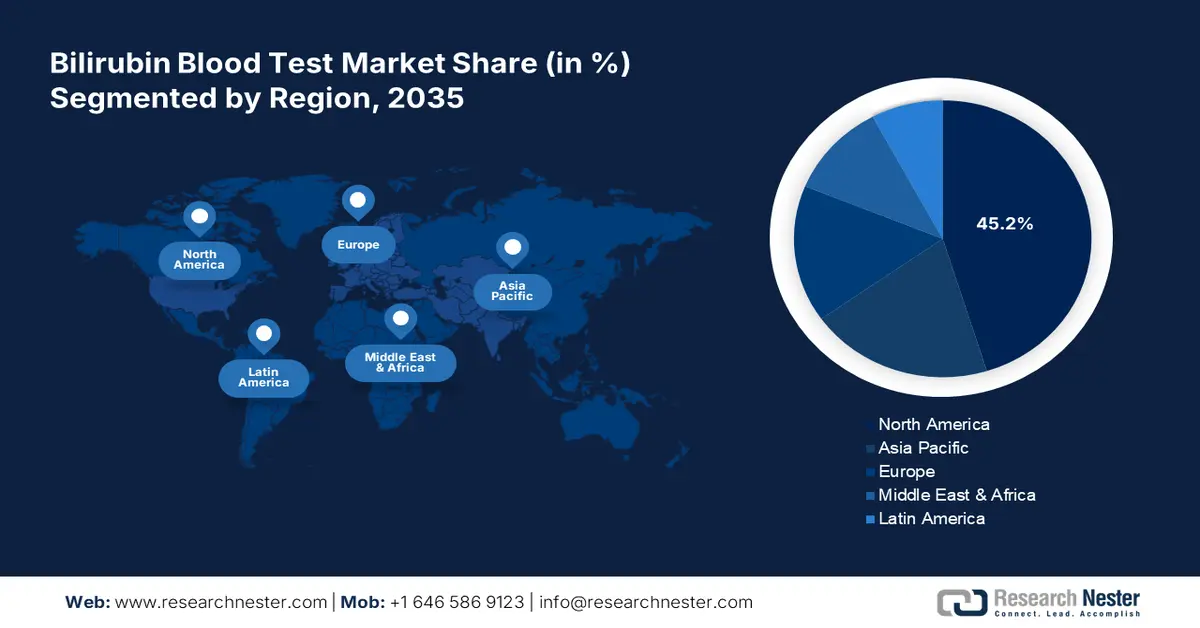
- North America dominates the Bilirubin Blood Test Market with a 45.00% share, driven by rising prevalence of liver diseases and increased preventive healthcare efforts, positioning it for growth through 2035.
- Asia Pacific’s bilirubin blood test market is forecasted to see lucrative growth by 2035, driven by healthcare infrastructure investments and government initiatives for early detection.
Segment Insights:
- The Infants segment is expected to capture over 65.2% market share by 2035, driven by the high prevalence of neonatal jaundice and the need for early detection.
- The consumables segment of the Bilirubin Blood Test Market is anticipated to maintain a majority of revenue share from 2026-2035, driven by recurring demand in diagnostic procedures and the necessity for regular replenishment.
Key Growth Trends:
- Preventive healthcare and lifestyle risks
- Supportive government policies
Major Challenges:
- High cost of advanced testing devices
- Regulatory and approval barriers
- Key Players: Beckman Coulter, Inc., Thermo Fisher Scientific Inc., Randox Laboratories Ltd., Hoffmann-La Roche AG.
Global Bilirubin Blood Test Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 1.57 billion
- 2026 Market Size: USD 1.66 billion
- Projected Market Size: USD 2.87 billion by 2035
- Growth Forecasts: 6.2% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (45.2% Share by 2035)
- Fastest Growing Region: North America
- Dominating Countries: United States, Germany, Japan, United Kingdom, France
- Emerging Countries: China, India, Brazil, Russia, Mexico
Last updated on : 13 August, 2025
Bilirubin Blood Test Market Growth Drivers and Challenges:
Growth Drivers
- Preventive healthcare and lifestyle risks: With the global shift toward preventive healthcare, bilirubin blood tests have become a standard component of diagnostic evaluations, facilitating early detection of liver-related conditions. According to a report published in March 2024, spending on preventive healthcare in the EU increased by 88.2% in current prices between 2020 and 2021, raising GDP from 0.38% to 0.65%. This proactive approach leads to improved patient outcomes and helps reduce healthcare costs. This trend has further driven the demand for bilirubin testing as a crucial tool for monitoring and managing liver health.
- Supportive government policies: Globally, governments are investing more in healthcare infrastructure to improve diagnostic capabilities and encourage the early detection of various health conditions. According to the International Trade Administration, the healthcare industry of India surpassed USD 370 billion in 2022. As part of these efforts, there is a growing emphasis on the use of bilirubin blood tests, which are vital for diagnosing liver and bile duct diseases. Additionally, subsidized healthcare programs and reimbursement policies make these tests more affordable and accessible, encouraging broader adoption by healthcare providers and patients, and ultimately improving overall public health outcomes.
Challenges
- High cost of advanced testing devices: Despite technological advancements in bilirubin testing, many portable and non-invasive devices come with high upfront costs, making them less accessible to low-resource settings or smaller healthcare facilities. These high costs can hinder the widespread adoption of such devices, especially in developing regions or underserved areas where budget constraints are more significant. As a result, healthcare providers rely on traditional, more affordable but less convenient methods, limiting access to timely and accurate diagnostics for liver-related conditions.
- Regulatory and approval barriers: The bilirubin blood test market faces significant regulatory challenges, as new testing technologies must undergo rigorous approval processes in different countries. These processes involve detailed evaluations to ensure the safety, effectiveness, and accuracy of diagnostic devices, which can be time-consuming and costly. As a result, the introduction of innovative devices, particularly non-invasive or home-use products, is often delayed. These regulatory barriers can slow bilirubin blood test market growth and limit the availability of cutting-edge solutions to healthcare providers and patients.
Bilirubin Blood Test Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
6.2% |
|
Base Year Market Size (2025) |
USD 1.57 billion |
|
Forecast Year Market Size (2035) |
USD 2.87 billion |
|
Regional Scope |
|
Bilirubin Blood Test Market Segmentation:
End user (Infants, Adults)
In bilirubin blood test market, infants segment is set to capture revenue share of over 65.2% by 2035. The segment’s growth of due to the high prevalence of neonatal jaundice, a common condition caused by immature liver function in newborns. Neonatal jaundice affects 60% of term and 80% of preterm newborns, with 1.1 billion developing hyperbilirubinemia annually worldwide as per NLM in January 2024. Early detection and monitoring of bilirubin levels are crucial to prevent complications, driving demand for these tests in neonatal care. Increased awareness among parents and healthcare providers, coupled with advancements in non-invasive testing technologies, has further boosted the adoption in the bilirubin blood test market.
Product Type (Instruments, Consumables)
In the bilirubin blood test market, by product type, the consumables segment is expected to garner the majority of revenue share attributed to their recurring demand in diagnostic procedures. Items such as reagents, test strips, and cartridges are essential for each diagnostic test, ensuring a consistent supply across healthcare facilities. Unlike diagnostic instruments, which typically involve a one-time investment, consumables require regular replenishment, making them a crucial source of ongoing revenue. Additionally, the rising global demand for a variety of tests further enhances the need for these consumables, solidifying their vital role and dominance in the market.
Our in-depth analysis of the global bilirubin blood test market includes the following segments:
|
End User |
|
|
Product Type |
|
|
Test Type |
|
|
Application |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Bilirubin Blood Test Market Regional Analysis:
North America Market Statistics
By 2035, North America bilirubin blood test market is predicted to dominate over 45.2% revenue share. The rising prevalence of liver diseases such as hepatitis, cirrhosis, and fatty liver disease in North America is driving demand for bilirubin blood tests, as these conditions require regular monitoring. In August 2023, the Journal of Hepatology reported that 24-48% of people in North America have nonalcoholic fatty liver disease (NAFLD). Governments promote preventive healthcare by supporting bilirubin testing for liver disorders through enhanced accessibility and insurance coverage in routine assessments. Thus, propelling the bilirubin blood test market.
The U.S. healthcare system’s growing emphasis on preventive care and early disease detection has driven the adoption of bilirubin blood tests as a key tool for identifying liver conditions early. Government initiatives and policies promoting regular liver screenings have further supported this trend. Additionally, there has been an expansion of healthcare infrastructure. According to the American Medical Association, health spending in the U.S. increased by 4.1% in 2022, reaching USD 4.5 trillion, or USD 13,493 per capita. The growth of advanced diagnostic labs and outpatient centers has improved access to bilirubin testing across the country, particularly in rural and underserved areas, contributing to the expansion of the bilirubin blood test market.
The aging population in Canada is more prone to liver-related diseases such as cirrhosis and liver cancer, which is driving the demand for regular bilirubin blood tests to monitor liver health. With an increasing number of elderly individuals requiring routine liver checks, the need for accurate diagnostic tools grows. Additionally, a heightened awareness of liver health and lifestyle factors such as obesity, alcohol consumption, and poor diet is prompting the population in Canada to seek liver function tests, further boosting the demand for bilirubin testing and, in turn propagating the bilirubin blood test market.
Asia Pacific Market Analysis
In APAC, the bilirubin blood test market is poised to garner lucrative market share over the forecast period. Many Asia Pacific countries are investing in healthcare infrastructure and promoting early disease detection, particularly for liver conditions. Government initiatives focused on preventive healthcare, regular liver screenings, and reimbursement for diagnostic tests are making bilirubin blood tests more affordable and accessible. This, combined with the rapid expansion of healthcare infrastructure in developing regions such as India, China, and Southeast Asia, has improved access to advanced diagnostic facilities, driving wider adoption of bilirubin testing, especially in rural and underserved areas.
The government in China has significantly invested in healthcare infrastructure, focusing on early disease detection and prevention through initiatives such as subsidized healthcare and regular liver screenings. For instance, as published in BioMed Central Ltd in September 2024, the government of China invested USD 268.8 billion in healthcare in 2020. These efforts have increased the adoption of bilirubin blood tests, especially in rural areas, where access to medical services is improving. Additionally, reforms in healthcare reimbursement policies have made diagnostic tests, including bilirubin testing, more affordable. These policies encourage regular liver screenings and diagnostic services, driving higher demand for diagnostic services, and driving higher demand for the bilirubin blood test market across healthcare settings.
The development of advanced, non-invasive bilirubin testing devices has made testing easier and more affordable in India. Point-of-care devices provide faster results with minimal discomfort and are increasingly used in rural healthcare centers. The AJO-Neo device, developed in Kolkata, allows for rapid screening of neonates, improving detection time and reducing the risk of kernicterus as per the Ministry of Science & Technology in July 2020. Additionally, medical reimbursement policies like Ayushman Bharat have lowered diagnostic costs, boosting adoption in both public and private healthcare settings. Thus, propelling the bilirubin blood test market.
Key Bilirubin Blood Test Market Players:
- Koninklijke Philips N.V.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Beckman Coulter, Inc.
- Thermo Fisher Scientific Inc.
- Randox Laboratories Ltd.
- Hoffmann-La Roche AG
- Drägerwerk AG & Co. KGaA
- Ginevri
- Advanced Instruments
- Reichert, Inc.
- Instrumentation Laboratory.
- AVI Healthcare Pvt. Ltd.
- Ipsen
- Athenese-Dx
Key companies in the bilirubin blood test market are heavily investing in research and development to introduce advanced, non-invasive, and portable testing devices. They are focusing on enhancing diagnostic accuracy and patient comfort, particularly for neonatal and point-of-care applications. Collaborations with healthcare providers and government initiatives further support these advancements. For instance, in September 2023, IIT Kanpur partnered with Sensa Core Medical Instrumentation to mass-produce innovative point-of-care bilirubin analyzers, boosting accessibility and growth in the market. Additionally, companies are expanding their product portfolios by upgrading manufacturing capabilities.
Some of these key players in bilirubin blood test market:
Recent Developments
- In November 2024, Ipsen presented phase III data showing Bylvay’s sustained efficacy in PFI and ALGS, improving patient outcomes, and indirectly driving demand for bilirubin blood tests to monitor liver health.
- In April 2024, Athenese-Dx launched the TRUEchemie Bilirubin Total & Direct Test Kit offers a 30-month shelf life for accurate bilirubin analysis in serum, enhancing reliability and growth in the bilirubin blood test market.
- Report ID: 6974
- Published Date: Aug 13, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Bilirubin Blood Test Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




