Atrial Fibrillation Market Outlook:
Atrial Fibrillation Market size was over USD 30 billion in 2025 and is estimated to reach USD 75.5 billion by the end of 2035, expanding at a CAGR of 10.8% during the forecast timeline, i.e., 2026-2035. In 2026, the industry size of atrial fibrillation is estimated at USD 33.2 billion.
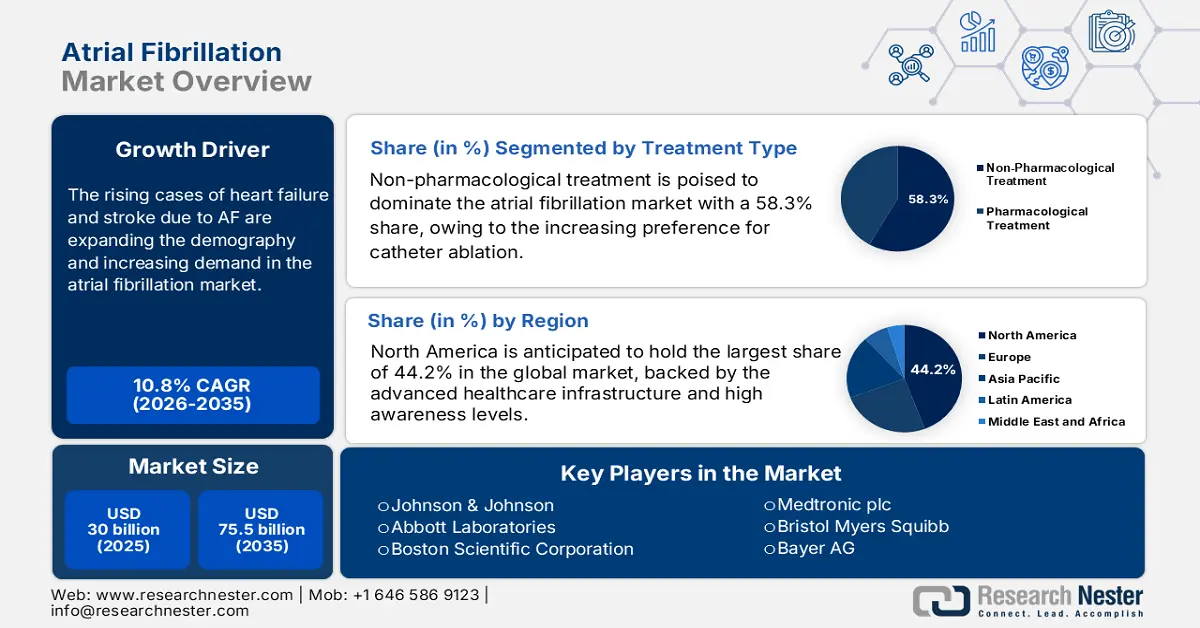
The rising cases of heart failure and stroke due to atrial fibrillation (AF) are creating a need for early detection and intervention to prevent death or disability among patients. Besides, the increasing age and lifestyle-related comorbidities and risks, including obesity, hypertension, and diabetes, are pushing both public and private healthcare service providers to procure maximum resources to support the afflicted population. Testifying to the enlarging epidemiology in the atrial fibrillation market, a study published in June 2023 unveiled that AF affected approximately 33 million individuals worldwide, becoming one of the primary causes of coronary heart disease (CHD) and deaths. Another NLM estimation projected the absolute global burden of this ailment to increase by over 60% by 2050.
As the demand for diagnostic, therapeutic, and surgical solutions to combat this widespread condition grows, the global trade of medical-grade raw materials, APIs, and precision manufacturing gains higher activity and engagement. However, the inflation in the payers’ pricing of consumer services and distributed products remains a roadblock for broad accessibility in the atrial fibrillation market. This can be evidenced by the 2025 study conducted by the Hebei Medical University, which unveiled that the annual direct cost of AF in the U.S. ranged between USD 2 thousand and USD 14.2 thousand. It also highlighted the per-patient per-year expenses on the same aspect reached USD 525.2-3501.6 in Europe. In response, pioneers in this sector are forming an alignment with value-based clinical models, enacted by public authorities.
Key Atrial Fibrillation Market Insights Summary:
Regional Insights:
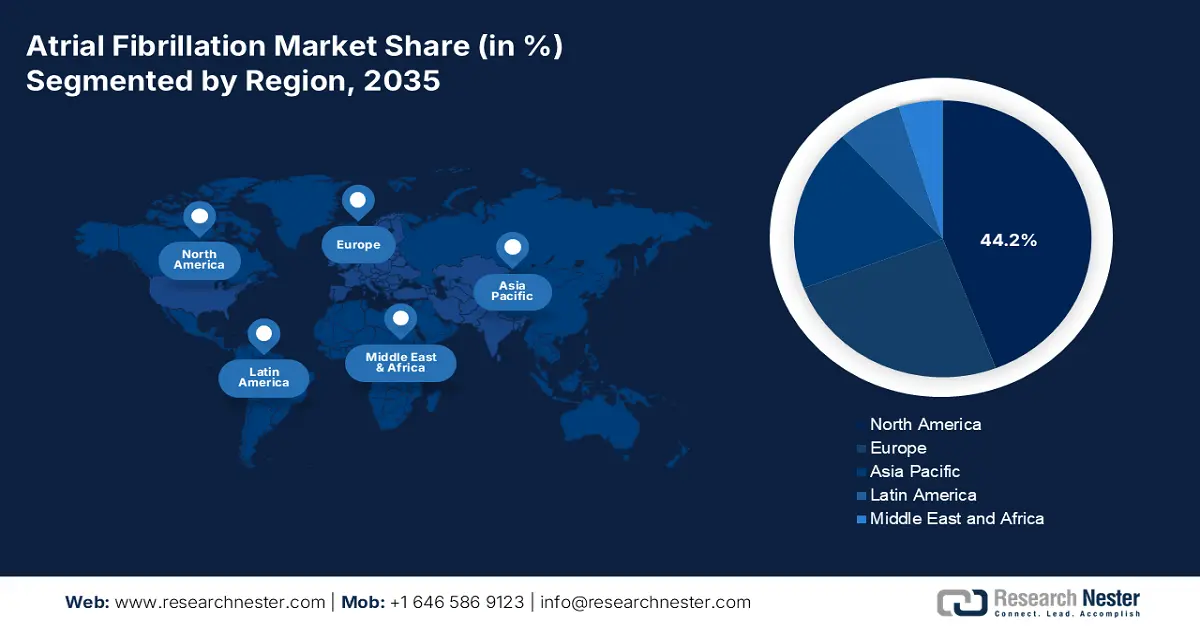
- North America is projected to hold a 44.2% share by 2035, impelled by advanced healthcare infrastructure and broad treatment availability in the atrial fibrillation market.
- Asia Pacific is anticipated to register the highest CAGR over 2026–2035, fueled by rising cardiovascular disease mortality and government-backed healthcare initiatives.
Segment Insights:
- Non-pharmacological treatment is projected to account for 58.3% share by 2035, owing to the increasing preference for catheter ablation procedures.
- Paroxysmal atrial fibrillation (PAF) is anticipated to hold a 51.2% share by 2035, propelled by early detection and intervention strategies.
Key Growth Trends:
- Continuous expansion in the geriatric population
- Growing trend of regular health monitoring
Major Challenges:
- Price caps and cost-containment policies
- Heterogeneity among regulatory pathways
Key Players:Johnson & Johnson (Biosense Webster), Abbott Laboratories, Boston Scientific Corporation, Medtronic plc, Bristol Myers Squibb, Bayer AG, Pfizer Inc., Siemens Healthineers AG, GE Healthcare, Koninklijke Philips N.V., Biotronik SE & Co. KG, iRhythm Technologies, Inc., MicroPort Scientific Corporation, Lepu Medical Technology (Beijing) Co., Ltd., OSYPKA AG, APN Health, LLC, ACS Diagnostics.
Global Atrial Fibrillation Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 30 billion
- 2026 Market Size: USD 33.2 billion
- Projected Market Size: USD 75.5 billion by 2035
- Growth Forecasts: 10.8% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (44.2% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, Japan, France, United Kingdom
- Emerging Countries: China, India, Brazil, South Korea, Australia
Last updated on : 11 September, 2025
Atrial Fibrillation Market - Growth Drivers and Challenges
Growth Drivers
- Continuous expansion in the geriatric population: According to a study published by the Journal of Clinical Medicine Research (JOCMR) in March 2023, a cross-sectional analysis on a selected patient pool observed an 8.9% higher AF occurrence among adults aged 80 years or older, in comparison to people aged 55 years or younger. It also highlighted that 70% of the afflicted residents in the U.S. were aged between 65 and 85. This indicates the substantial characteristics of the consumer base of the atrial fibrillation market, as the population across the world is aging rapidly. In this regard, the WHO estimated the global count of individuals aged 60 and over to cross 2.1 billion by 2050, 426 million of them are poised to be aged 80 or older.
- Growing trend of regular health monitoring: Increasing efforts from healthcare providers, advocacy groups, and industry players to raise public awareness of AF symptoms and risks are potentially expanding the demography of the market. Besides, the growing adoption of smartwatches, ECG patches, and mobile health apps that feature real-time heart monitoring is also influencing people to invest in disease prevention before it worsens. This ultimately demonstrates a new pathway of revenue generation in this sector. Besides, early diagnosis helps the commercially available therapeutic and interventional treatments deliver better results, establishing a stronger foundation for the merchandise.
- Introduction of novel and next-generation products: Increasing R&D investment and continuous innovations are underscoring a shift in the atrial fibrillation market from traditional solutions to revolutionary commodities. Moreover, the improving safety profile of drugs, efficacy of surgical tools, and affordability of medical equipment are influencing both consumers and pioneers to invest more in this field. Particularly, the wave of digitalization across the healthcare industry is enhancing patient satisfaction in this category. To capitalize on this trend, in August 2023, GE HealthCare launched a digital tool, CardioVisio, which provides assistance to clinicians in decision-making for AF, with its capability to analyze up-to-date guidelines and relevant longitudinal data.
Historic Regional and Socio-Demographic Trends in Global Burden of Disease (GBD)
Global and SDI-Level Trends in AF Burden (1990-2019)
|
Region/SDI Level |
Trend in AF Cases |
Most Notable Metric Changes |
Key Notes / Statistics |
|
High-Middle SDI |
Decreasing |
ASIR: -0.12 (95% CI: -0.15 to -0.09) ASMR: -0.25 (-0.32 to -0.18) ASDR: -0.25 (-0.28 to -0.23) |
Only the SDI group with declining AF burden metrics |
|
High-Income North America |
Increasing (since 2019) |
Highest ASIR: 108.53 (87.59-131.44) |
Also showed the most prominent increases in ASIR and ASDR |
|
Australasia (e.g., Australia) |
High mortality and DALY |
ASMR: 6.86 (5.45-8.33) ASDR: 168.28 (132.54-214.32) |
Among the highest in global AF-related mortality and disability |
Source: Frontiers
Legends:
- SDI = Sociodemographic Index
- DALY = Disability-Adjusted Life Year
- ASIR = Age-Standardized Incidence Rate
- ASMR = Age-Standardized Mortality Rate
- ASDR = Age-Standardized DALY Rate
- CI = Confidence Interval
Trends in Commercial Moves, Clinical Trials, and R&D Expanding the Atrial Fibrillation Drugs Market
Recent Commercial Launches and Clinical Developments in AF Drugs
|
Timeline |
Company |
Drug/Program |
Stage/Type |
Indication |
Key Details |
|
November 2023 |
Bayer |
Asundexian (BAY2433334) |
Phase III (OCEANIC-AFINA) |
AF patients ≥65 at high risk of stroke or systemic embolism and unsuitable for OACs |
Third Phase III study under the OCEANIC program; targets patients with high bleeding risk |
|
March 2023 |
Bristol Myers Squibb & Janssen |
Milvexian |
Phase III (Librexia Program) |
Oral Factor Xia inhibitor for antithrombotic treatment |
Collaborative Phase III study targeting the prevention of thrombotic events, including AF patients |
|
January 2023 |
Anthos Therapeutics |
Abelacimab |
Phase III (LILAC-TIMI 76) |
High-risk AF patients are unsuitable for current anticoagulants |
First patient enrolled; study focuses on safety and efficacy in patients not eligible for standard OACs |
|
September 2022 |
Anthos Therapeutics |
Abelacimab |
FDA Fast Track Designation |
Prevention of stroke/systemic embolism in AF patients |
Accelerated development granted by the FDA for a high-priority unmet clinical need |
|
June 2022 |
Apotex Inc. |
APO-Apixaban (generic) |
Commercial Launch |
Prevention and treatment of blood clots (e.g., in AF) |
First generic version of Eliquis in Canada; available in 2.5mg and 5mg tablets |
|
January 2022 |
Eagle Pharmaceuticals/AOP Orphan Pharma |
Landiolol |
Pre-NDA Regulatory Engagement |
Short-term reduction of ventricular rate in patients with supraventricular tachycardia, including AF/flutter |
Engaged with the FDA to define pre-clinical and clinical data requirements for NDA approval in the U.S. |
Source: Company Press Releases
Challenges
- Price caps and cost-containment policies: The efforts from governing bodies to make diagnosis, treatment, and prevention more accessible for eligible patients often create limitations in securing profit margins from the atrial fibrillation market. Particularly, national health systems use reference pricing and Health Technology Assessment (HTA) bodies to enforce strict price controls, which may cause financial loss for premium-priced brands. Moreover, the prioritization of cheaper alternatives is discouraging global leaders from investing in innovation.
- Heterogeneity among regulatory pathways: Regulatory criteria for approval vary notably across key landscapes, including the U.S., Japan, and Europe. As a result, a product from the market may face delays of 12-24 months due to differing clinical data requirements, trial design standards, and bureaucratic processes. For instance, in 2022, new clinical trial regulations for target arrhythmias and ablation procedures were enacted by the Japanese Catheter Ablation (J‐AB) registry that required additional real-world evidence for implantable devices, even after being approved by others.
Atrial Fibrillation Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
10.8% |
|
Base Year Market Size (2025) |
USD 30 billion |
|
Forecast Year Market Size (2035) |
USD 75.5 billion |
|
Regional Scope |
|
Atrial Fibrillation Market Segmentation:
Treatment Type Segment Analysis
Non-pharmacological treatment is poised to dominate the atrial fibrillation market with a 58.3% share by the end of 2035. The leadership is highly attributable to the increasing preference for catheter ablation procedures as a highly effective option for patients who are resistant to or intolerant of drug therapy. Moreover, having the potential for long-term rhythm control by directly targeting the electrical pathways causing AF, these tools receive wide acceptance and utilization to reduce symptoms and improve quality of life. Exemplifying the same, in October 2024, Boston Scientific earned FDA approval and 510(k) clearance for its navigation-enabled FARAWAVE NAV Ablation Catheter and new FARAVIEW Software for the treatment of paroxysmal AF.
Application Segment Analysis
Paroxysmal atrial fibrillation (PAF) is estimated to represent itself as the largest field of application in this sector over the assessed period, while accounting for around 51.2% share. Being the initial stage of the primary condition, PAF makes it necessary to enable early detection and intervention. Thus, both treatment approaches, including catheter ablation and targeted therapeutics, are increasingly focused on this category to prevent progression to persistent or permanent AF. Besides, the ongoing advances in monitoring technologies and diagnostic tools are improving the detectability of this subtype, making management of PAF faster and more convenient.
End user Segment Analysis
Hospitals and cardiac centers are predicted to remain the leading end users in the atrial fibrillation market till the end of the discussed timeframe by securing a 75.5% revenue share. These facilities are equipped with specialized infrastructure and skilled electrophysiologists, which are necessary to perform complex AF interventions, including catheter ablation and device implantation. This enables a substantial patient pool for this segment, contributing to steady cash inflow in this sector. Furthermore, the rising number of AF patients requiring advanced diagnostic and therapeutic procedures is also amplifying demand for services available in hospital settings.
Our in-depth analysis of the atrial fibrillation market includes the following segments:
|
Segment |
Subsegments |
|
Treatment Type |
|
|
Product |
|
|
Application |
|
|
End user |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Atrial Fibrillation Market - Regional Analysis
North America Market Insights
North America is anticipated to hold the largest share of 44.2% in the market throughout the analyzed tenure. The advanced healthcare infrastructure, high awareness levels, and availability of broad treatment options are solidifying the region’s forefront position in this sector. The aging population and a growing occurrence of lifestyle-related risk factors, such as obesity and hypertension, are also contributing to the demographic expansion of AF, garnering a surge in cutting-edge technologies and novel supplements. The landscape’s upward augmentation is further supported by the presence of favorable reimbursement policies and globally renowned clinical organizations.
According to the NLM, in 2025, approximately 10.5 million adults in the U.S. were living with AF, accounting for approximately 5% of the total population. Another report from the Health Action Council revealed that every 2 in 5 adults and 1 in 5 children in the country were obese till 2025. This indicates continuous enlargement of the consumer base for novel therapies and efficient interventional solutions, ultimately benefiting the market. The country is further fueling the sector with a steady cash inflow through its comprehensive insurance coverage, fostering a greater scope for innovation in this field.
Canada is propagating the market steadily on account of its publicly funded healthcare system that ensures broad access to diagnostic and treatment services. The country’s strong focus on serving the elderly population is also creating a substantial demand base for effective AF management strategies. However, the country’s significance in this sector is moderated by budget constraints and longer approval timelines compared to the U.S., where the governing bodies are actively promoting localized advancement of technologies and improvement of care pathways to bridge this gap.
APAC Market Insights
Asia Pacific is expected to exhibit the world's highest CAGR in the atrial fibrillation market over the selected timeline. The increasing mortality of cardiovascular diseases (CVD) and government-backed healthcare spending are propelling the region’s progress in this sector. Particularly, emerging economies, such as China and India, are prompting greater demand for both pharmacological and non-pharmacological treatments. This is attracting pioneers to invest and participate in this landscape. This can be exemplified by Johnson & Johnson’s expanded launch of the VARIPULSE Platform across APAC countries, including Japan, Hong Kong, China, Australia, Taiwan, and Korea, in July 2025.
Notable growth medical device industry is the major driver behind the robust expansion of the China market. As access to advanced procedures and therapeutics for patients improves, the country is fostering a favorable atmosphere for widespread adoption in this sector. Additionally, government initiatives focused on stroke prevention and chronic disease management are accelerating early diagnosis and intervention. Such public support can be testified by a 2024 NLM report, unveiling that 58% of the total stroke-related medical costs in China are covered by social health insurance, and 14% were reimbursed by government sources.
India is becoming an important growth factor for the regional market, which is primarily driven by a rising occurrence of lifestyle-related risk factors such as hypertension, diabetes, and obesity. On the other hand, as the country’s population continues to age, AF cases are expected to increase notably in India, creating a sustainable consumer base for the merchandise beyond urban centers. Moreover, ongoing government-led initiatives to improve rural healthcare accessibility and complete the modernization of the national medical system are collectively expanding the sector’s reach nationwide.
Country-wise Trends in the AF Patient Population
|
Country |
Key Notes |
Timeline |
|
China |
Mortality rate of CVDs accounts for 46.74%–44.26% of all deaths in rural and urban areas |
2024 |
|
India |
Crude incidence of stroke ranged from 108 to 172 per 100,000 people per year; One-month case fatality rates range from 18% to 42% |
2021 |
|
Australia |
Ranked 5th most prevalent country for AF globally; More than 600 thousand individuals are expected to be diagnosed with AF by 2034 |
2024-2034 |
Source: Frontiers, NLM, and ScienceDirect
Europe Market Insights
Europe is predicted to remain the second-largest shareholder with presenting a mature and progressive landscape for the global atrial fibrillation drugs market during the timeline between 2026 and 2035. The region’s consistent growth in this sector is primarily driven by an enlarging geriatric population and growing emphasis on the MedTech industry. Besides, the empowering medical system across the region support widespread access to advanced AF therapies, including novel oral anticoagulants (NOACs) and antiarrhythmic drugs. Moreover, the well-established clinical guidelines and proactive screening programs promote early diagnosis and effective management.
The UK is fueling growth in the Europe atrial fibrillation market with a growing emphasis on early diagnosis and stroke prevention measures. The National Health Service (NHS) is playing a pivotal role in this cohort of spreading awareness by implementing robust screening and management guidelines for AF, particularly for the therapeutic consumption. On the other hand, the UK is proactively participating in R&D and innovation in electrophysiology, which is bringing revolutionary advances to this sector. Moreover, the amplified investment in digital health initiatives and chronic disease management campaigns is consolidating the country’s position in this field.
Germany is one of the biggest and most influential contributors to the Europe atrial fibrillation drugs market. The country’s advanced healthcare system and rapidly aging population are collectively fueling demand in this sector. Thus, with such a high occurrence of AF, nationwide clinical institutions and government authorities are investing heavily in extensive research and development in this category. As evidence, in January 2025, a team of researchers at the DZHK Munich site, working in alliance with colleagues from the USA, France, and the Netherlands, was awarded USD 8 million over five years by the Leducq Foundation. This funding was assigned to indentifying immune cell roles in AF with an aim to develop targeted therapies.
Feasibility Opportunities for the AF Drugs Market
|
Country |
Key Notes |
Timeline |
|
UK |
30% of patients after cardiac surgery suffer from postoperative AF |
2024 |
|
Germany |
Asundexian showed the potential to secure peak sales of more than USD 5.8 billion |
2023 |
|
Switzerland |
The incidence of AF is estimated to double by the end of 2060 |
2018-2060 |
Source: NLM, Company Press Release, and ESC Congress
Key Atrial Fibrillation Market Players:
- Johnson & Johnson (Biosense Webster)
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- Abbott Laboratories
- Boston Scientific Corporation
- Medtronic plc
- Bristol Myers Squibb
- Bayer AG
- Pfizer Inc.
- Siemens Healthineers AG
- GE Healthcare
- Koninklijke Philips N.V.
- Biotronik SE & Co. KG
- iRhythm Technologies, Inc.
- MicroPort Scientific Corporation
- Lepu Medical Technology (Beijing) Co., Ltd.
- OSYPKA AG
- APN Health, LLC
- ACS Diagnostics
The atrial fibrillation market is becoming increasingly competitive, with both established and emerging players expanding their presence through innovation. Exemplifying the same, in November 2024, AOP Orphan Pharmaceuticals received FDA approval for its fast-acting treatment, Rapiblyk (landiolol), for AF and flutter in critical care settings. This marked AOP Health’s entry into the U.S. market and added a new option for hospital-based heart rate control with minimal blood pressure impact. The approval also reflects growing competition in acute AF management, as companies continue to do research in both pharmacological and interventional therapies.
Here is a list of key players operating in the market:
Recent Developments
- In May 2025, Abbott launched a new ablation catheter, TactiFlex Sensor Enabled, in India, featuring a flexible tip and contact force technology. This is designed to function along with the company’s EnSite X EP System to offer a better visualization for physicians, while lowering procedure times and enabling greater safety.
- In February 2025, Kardium raised USD 250 million in a new financing round for developing and commercializing its innovative AF treatment, Globe Pulsed Field System. This empowered the company’s plans to expand its pipeline worldwide with higher manufacturing and marketing capabilities.
- Report ID: 8093
- Published Date: Sep 11, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Atrial Fibrillation Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




