Atorvastatin API Market Regional Analysis:
North America Market Statistics
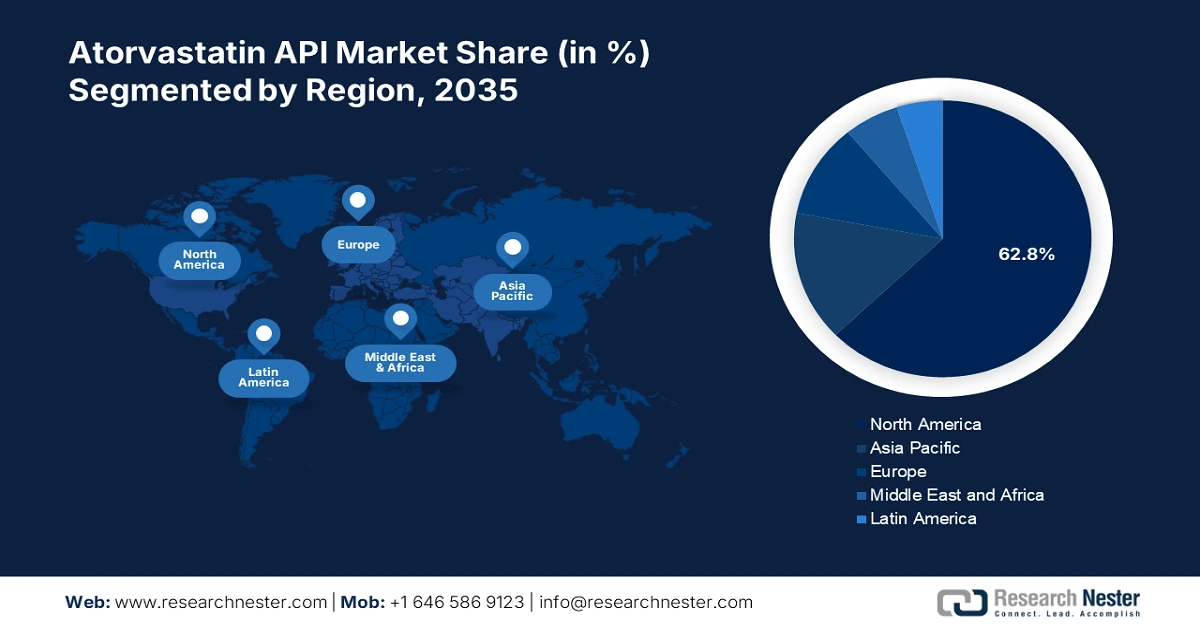
North America in atorvastatin API market is likely to hold over 62.8% revenue share by the end of 2035. The need for efficient cholesterol-lowering treatments has increased due to the rising prevalence of lifestyle-related illnesses such as diabetes and obesity. Pharmaceutical firms in the region have been enticed by this situation to invest in the creation and production of atorvastatin APIs, which has allowed them to diversify their product lines and improve patient access to necessary drugs.
Canada atorvastatin API market is anticipated to grow rapidly during the forecast period owing to the expanding infrastructure in research and development of API treatments. For instance, in July 2022, Piramal Pharma Limited's Pharma Solutions business inaugurated a new API plant. The facility is located within the business' headquarters in Aurora, Ontario. As one of the leading Contract Development and Manufacturing Organizations (CDMO), its objective is to have quality medications available in the market.
The U.S. atorvastatin API market is growing significantly attributable to the support of government agencies and organizations in the development and approvals of novel drugs. For instance, in August 2020, Biocon Biologics India Ltd. and Mylan N.V. announced the U.S. launch of Semglee in vial and pre-filled pen presentations. The U.S. Food and Drug Administration (FDA) has confirmed that Semglee is approved for the same indications as Sanofi's Lantus and shares the same amino acid sequence. Thus, this motive leads to preparing a conducive environment for treatments.
Asia Pacific Market Analysis
The atorvastatin API market in Europe is gaining traction and is expected to witness lucrative growth during the forecast timeline. Producers in the region are able to reduce production costs and guarantee consistent product quality owing to innovations such as continuous manufacturing and advancements in synthesis techniques. These technological developments are crucial for ensuring compliance with increasingly strict regulations while satisfying the rising demand for atorvastatin worldwide. This market shift is indicative of a larger movement in pharmaceutical manufacturing toward increased quality and efficiency.
China atorvastatin API market is experiencing significant growth owing to the rising number of people suffering from chronic diseases. According to IDF's 2022 statistics, 141 million people in China had diabetes in 2021. The high diabetic and obese population in the country has led to a stronger focus on developing advanced and safe API drugs. In addition, the WHO analysis states that the China government shifted its economy from centrally planned to market-based for the expansion and establishment of API manufacturing facilities in the country. For instance, in June 2022, WuXi STA opened a brand-new facility for a high-potency active pharmaceutical component at its location in Jiangsu. The firm expanded its Changzhou facility in response to increased demand for high-potency API process research and development services.
India is unfolding innumerable growth opportunities in the atorvastatin API market owing, the atorvastatin's patents have expired, and generic versions have proliferated, drastically reducing costs and expanding availability. Atorvastatin APIs' customer base is growing and competition is intensifying due to the generic market's expansion. Presence of leading producers of generic drugs worldwide, accounting for 20% of the world's generic medicine demand by volume. According to an Invest India Article, published in January 2023, the API industry of India held the 3rd leading position globally, with around 57% of WHO prequalified APIs.