Antibody Validation Market Outlook:
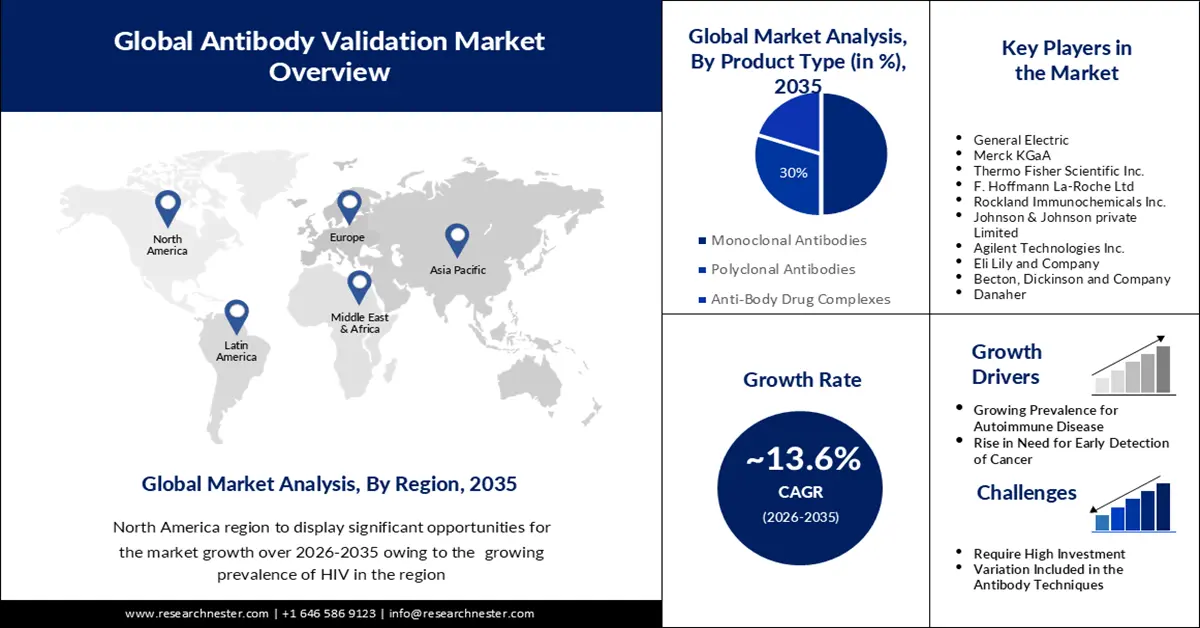
Antibody Validation Market size was over USD 476.39 billion in 2025 and is projected to reach USD 1.71 trillion by 2035, witnessing around 13.6% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of antibody validation is evaluated at USD 534.7 billion.
This growth of the market is anticipated to be influenced on account of growing cases of COVID-19. A large number of people were found to have symptoms of COVID-19 but were not been tested. Hence, due to this, the COVID-19 antibody validation demand was boosted. For instance, to treat COVID-19, an estimated 70 monoclonal antibodies are currently being developed or are through clinical studies.
Moreover, similar to COVID-19 there are numerous other diseases that are set to spread among people. These diseases include Ebola, Marburg virus disease, Lassa fever, MERS-CoV, SARS, and more. However, most of these diseases have no vaccine and research is yet to be conducted. These diseases are referred to as "Disease X," a pandemic-causing sickness that would never have been encountered by mankind before. COVID-19 has been identified as Disease X, prompting scientists to rush to discover therapies and vaccinations to combat it. Hence, in order to avoid the covid19 situation in the coming years, the demand for antibody validation is growing.