Albinism Drugs Market Outlook:
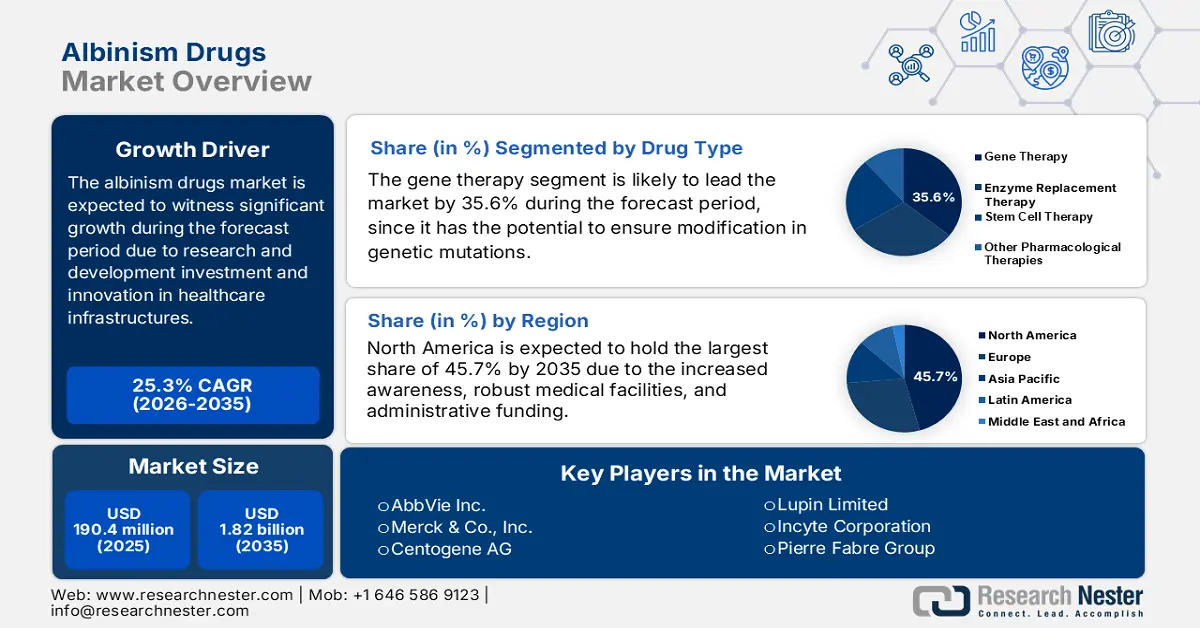
Albinism Drugs Market size was valued at USD 190.4 million in 2025 and is expected to reach USD 1.82 billion by 2035, expanding at around 25.3% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of albinism drugs is evaluated at USD 233.75 million.
The market has gained increased popularity owing to an increase in patient effectiveness, driven by advanced diagnostic solutions and awareness. According to the World Health Organization (WHO) report, albinism usually affects 1 in 20,000 people internationally, with an increased incidence rate of 1 in 1,000 in sub-Sahara region of Africa. In addition, the overall population affected by albinism is estimated to be 2 million people across nations. Hence, with enlarged incidences in particular areas, the market demand is anticipated to strengthen depending on targeted therapies and symptomatic treatment solutions through research and development to meet demographic requirements.
Moreover, the albinism drugs market development is fueled by supply chain activities, including medical devices and active pharmaceutical ingredients (APIs), and raw materials sourced from nations that comprise robust pharma manufacturing abilities, especially in the United States, India, and China. As per the U.S. FDA report, more than 80% of APIs are imported into the U.S. for drug production from specialized pharmaceutical manufacturing hubs. Besides, the U.S. Bureau of Labor Statistics data report stated a 2.5% yearly increase in the producer price index of pharma products, constituting continuous innovation. Also, the consumer price index for the same experienced a surge by 3.2% yearly, thus positively impacting the albinism drugs market growth.
Key Albinism Drugs Market Insights Summary:
Regional Highlights:
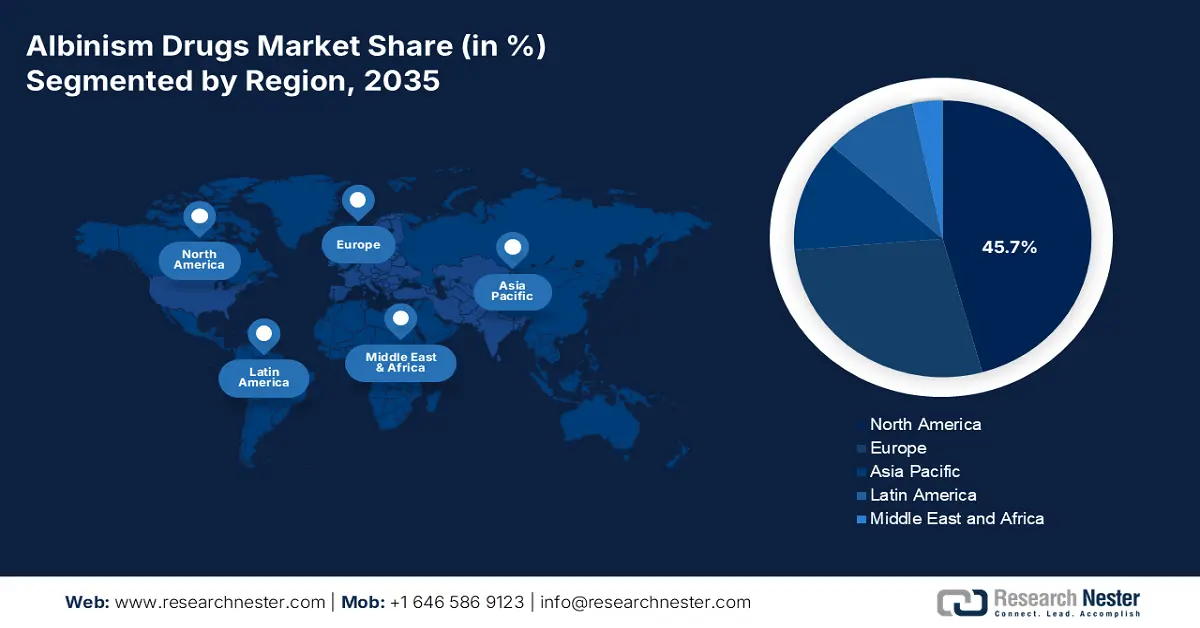
- By 2035, North America is expected to command a 45.7% share of the albinism drugs market, underpinned by supportive government strategies, rising disease awareness, and advanced healthcare infrastructure.
- Europe is projected to secure a 25.8% share by 2035, fostered by standard administrative policies, progress in genetics research, and increasing disease incidence.
Segment Insights:
- By 2035, the gene therapy segment is projected to account for a 35.6% share in the albinism drugs market, propelled by its capability to modify genetic mutations.
- Across 2026-2035, the genetic counselling and therapy segment is estimated to hold a 20.8% share, bolstered by its role in providing insights into inheritance patterns and genetic causes of albinism.
Key Growth Trends:
- Government funding for healthcare
- Improvement in health quality
Major Challenges:
- Pricing limitations leading to poor market accessibility
- Meticulous reimbursement policy for rare disease treatment
Key Players: Bayer AG, GlaxoSmithKline (GSK), Johnson & Johnson Services, AbbVie Inc., Merck & Co., Inc., Centogene AG, HumanOptics AG, Avita Medical, Laboratoires Genevrier, Allergan (AbbVie), Clinuvel Pharmaceuticals, Lupin Limited, Incyte Corporation, Pierre Fabre Group, Candela Corporation, Novartis AG, Bausch Health Companies Inc., Medytox Inc., Sun Pharmaceutical Industries, BioGenix Pharmaceuticals.
Global Albinism Drugs Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 190.4 million
- 2026 Market Size: USD 233.75 million
- Projected Market Size: USD 1.82 billion by 2035
- Growth Forecasts: 25.3%
Key Regional Dynamics:
- Largest Region: North America (45.7% Share by 2035)
- Fastest Growing Region: Europe
- Dominating Countries: United States, China, Japan, Germany, United Kingdom
- Emerging Countries: India, Brazil, South Korea, Mexico, Singapore
Last updated on : 3 December, 2025
Albinism Drugs Market - Growth Drivers and Challenges
Growth Drivers
- Government funding for healthcare: This factor plays a supportive role for research-based treatment accessibility in the case of rare diseases such as albinism. As per the Medicare.gov report, the U.S. government invested USD 12 billion to ensure suitable treatment procedures for albinism. In addition, national health systems in Europe expanded their budgets, with France's national health insurance endowment allocating EUR 350 million yearly for aiding genetic disorders. Therefore, the rising focus of government agendas and legislative strategies on rare disorders is facilitating the albinism drugs market evolution.
- Improvement in health quality: Quality improvision highlights positive outlooks of initial individual intervention with albinism. A clinical study conducted by the Agency for Healthcare Research and Quality (AHRQ) in 2022 proclaimed that vision-supporting drugs utilization in early stages tends to diminish the patient hospitalization rate by 25%. This resulted in saving healthcare expenditure by approximately USD 150 million within two years. Hence, such findings demonstrate long-term economic benefits of intervention, and drive health systems to make albinism treatment more effective and affordable.
Challenges
- Pricing limitations leading to poor market accessibility: This is one of the most significant barriers for the albinism drugs market to achieve entry in almost all nations. Besides, the existence of strict regulations is common in many nations, making it challenging for manufacturers to achieve profitability while initiating broad patient access. However, in Europe, organizations were able to combat price caps by partnering with national health agencies. This eventually resulted in a 10% upsurge in the market entry, which in turn, proved the public-private partnerships for combating price restrictions.
- Meticulous reimbursement policy for rare disease treatment: The presence of government healthcare programs, such as Medicaid and Medicare, often comprises severe repayment criteria. This makes it extremely problematic for patients suffering from the rare condition of albinism to access therapies without significant out-of-pocket costs. For instance, it was noted in 2023 that Medicare only covers 40% of the albinism treatment cost, making the drug expensive, especially for low and middle-income people, thus causing a hindrance in the albinism drugs market.
Albinism Drugs Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Year |
2026-2035 |
|
CAGR |
25.3% |
|
Base Year Market Size (2025) |
USD 190.4 million |
|
Forecast Year Market Size (2035) |
USD 1.82 billion |
|
Regional Scope |
|
Albinism Drugs Market Segmentation:
Drug Type Segment Analysis
Based on drug type, the gene therapy segment is projected to account for the largest share of 35.6% in the albinism drugs market by the end of 2035, owing to its capability to modify genetic mutations. In addition, the involvement of the government in providing generous funds for rare disorders and implementing biological treatments also readily impacts the segment’s upliftment. The unveiling of gene therapies through clinical trials and studies ensures effective and long-term solutions for patients, thus establishing a standard procedure to combat the occurrence of albinism.
Application Segment Analysis
Based on treatment type, the genetic counselling and therapy segment is estimated to hold a share of 20.8% in the albinism drugs market during the forecast period. The growth is subject to ensuring albinism management among patients with an insight into the inheritance and genetic cause of the disorder. According to a report published by the National Institutes of Health (NIH), genetic counselling comprises family planning options and diagnostic evaluation. Besides, the National Human Genome Research Institute (NHGRI) demonstrated that gene therapy has the potential to accurately produce genetic mutations at early stages. This offers a long-lasting solution to cater to genetically based defects, thus driving the segment’s growth.
Patient Type Segment Analysis
Based on patient type, the pediatric patients segment is anticipated to hold a considerable share of 15.5% in the albinism drugs market by the end of the forecast timeline. As stated in the National Institute of Child Health and Human Development (NICHD), albinism among children is naturally diagnosed early in life since the condition affects both the skin and eyes. This emphasizes regular eye evaluation since the visual impairment differs among different children. Additionally, the American Academy of Pediatrics (AAP) report demonstrated the essential responsibility of pediatricians to coordinate with genetic counselors and provide an all-inclusive care plan for children, thus positively impacting the segment.
Our in-depth analysis of the global albinism drugs market includes the following segments:
|
Drug Type |
|
|
Application |
|
|
Patient Type |
|
|
Treatment Type |
|
|
Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Albinism Drugs Market - Regional Analysis
North America Market Insights
The North America albinism drugs market is estimated to account for a share of approximately 45.7% by the end of 2035. The region’s growth is attributed to the availability of supportive government strategies, increased awareness among the population regarding the disease, and well-advanced healthcare facilities. Besides, countries such as the U.S. and Canada are gaining increased exposure by implementing innovative technology to assist both patients and medical professionals in tackling albinism by providing fruitful solutions.
The albinism drugs market in the U.S. is significantly growing due to disease awareness and budget provision to ensure an overall healthy lifestyle. For instance, in 2023, the country assigned 9.3% of its total health budget, which is estimated to be USD 5.1 billion, to ensure albinism drug availability. In addition, Medicaid also allocated USD 1.4 billion to introduce the newest treatment and enhance patient access in the country. Also, Medicare’s spending increased by 15.2%, that is USD 800.3 million, for the commercialization of albinism drugs, thus reflecting the market’s strength.
There is a huge growth opportunity for the albinism drugs market in Canada owing to the effective involvement of provincial and federal dignitaries. For instance, last year, the country allocated USD 3.5 billion, which caters to 8.5% of the federal healthcare budget, for the increased availability of albinism drugs. Besides, in Ontario, the public health system upsurged its investment by 18.1%, which benefited more than 200,100 patients yearly. Additionally, the Canada Institute for Health Information reported a spending of USD 18.6 billion by the public drug program, which significantly resulted in high-cost treatments, thereby boosting the market in the country.
Europe Market Insights
Europe albinism drugs market is predicted to hold the second-largest revenue share internationally, accounting for 25.8% during the forecast period. Factors such as suitable and standard administrative policies, innovation in genetics research, and increased disease incidence are readily responsible for the market’s upliftment in the region. Besides, the regulatory bodies, including the Europe Medicines Agency, have provided different orphan drugs, and the Europe Union initiated a fund of €2.5 billion for research in rare diseases. Therefore, all these factors and initiatives denote a prolific opportunity for the market to expand in the region.
The albinism drugs market in the UK is significantly growing since generous funding has been provided to overcome albinism. The presence of domestic and international regulatory organizations is deliberately making contributions to ensure the absence of the condition among the population in the country. For instance, the Association of the British Pharmaceutical Industry (ABPI) proclaimed that since 2021, there has been a 10.2% surge in the accessibility of albinism treatment. Therefore, with such development in the country, the market is poised to develop effectively.
The albinism drugs market in Germany is gaining increased exposure and is considered one of the largest markets in the whole of Europe. This is attributed to the increase in healthcare and medical spending, and the country has made an investment of €4.4 billion in 2024, which denotes a positive impact for amplifying the market. Moreover, the Federal Ministry of Health is also responsible for issuing funds to continuously carry out research and development activities in the country. Besides, the German Medical Association stated that 16 latest albinism treatment options have been introduced in the country, which are suitable to address patients’ needs to overcome the rare disease.
Albinism Drugs Market Players:
- Bayer AG
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- GlaxoSmithKline (GSK)
- Johnson & Johnson Services
- AbbVie Inc.
- Merck & Co., Inc.
- Centogene AG
- HumanOptics AG
- Avita Medical
- Laboratoires Genevrier
- Allergan (AbbVie)
- Clinuvel Pharmaceuticals
- Lupin Limited
- Incyte Corporation
- Pierre Fabre Group
- Candela Corporation
- Novartis AG
- Bausch Health Companies Inc.
- Medytox Inc.
- Sun Pharmaceutical Industries
- BioGenix Pharmaceuticals
Organizations in the albinism drugs market are adopting numerous strategies to augment their market position. Strategies, including research and development initiatives to launch and strengthen the product portfolio, expand their services, and focus on collaborations, strategic partnerships, are exceptionally handled by companies across all nations. Through these strategies, organizations provide diagnostic solutions, assist in addressing pigmentation diseases, manufacture regenerative medicines and standard medical devices, and launch personalized drugs. Besides, companies are continuously focusing on niche market areas to implement these strategies, which denotes a positive outlook for the market across nations.
Here is a list of key players operating in the global market:
Recent Developments
- In April 2024, Novartis unveiled a gene therapy for albinism, which focuses on amending genetic mutations accountable for albinism. Its early trials specified almost 40.2% improvement in visual acuity and pigmentation.
- In March 2024, BioGenix Pharmaceuticals introduced AlbiCure, which is the newest topical treatment, establishing a 25.3% enhancement in skin pigmentation after Phase 2 trials, heightening the organization’s share by 15.2%.
- Report ID: 7672
- Published Date: Dec 03, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Albinism Drugs Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




