Alagille Syndrome Market Outlook:
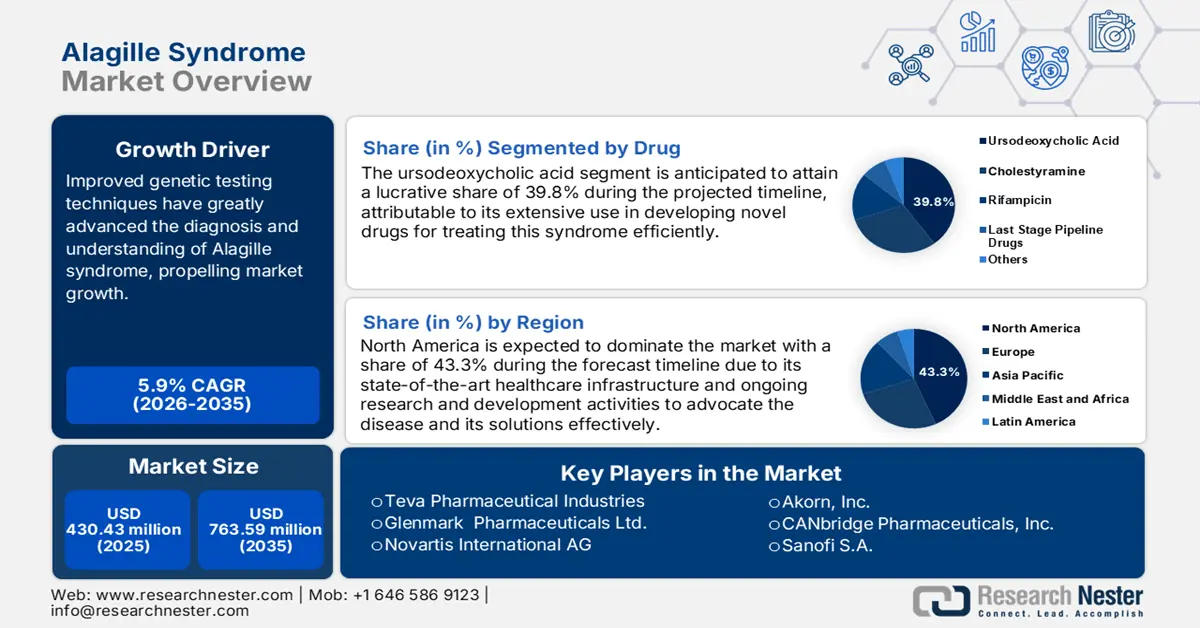
Alagille Syndrome Market size was over USD 430.43 million in 2025 and is anticipated to cross USD 763.59 million by 2035, growing at more than 5.9% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of alagille syndrome is assessed at USD 453.29 million.
Globally, 80% of rare diseases are inherent, and nearly 70% are present in children as reported by the Launcet Global Health in March 2024. Additionally, the report also estimated that 95% of rare diseases lack appropriate ailments and the standard time for precise diagnosis is 4 to 8 years. The incidence of alagille syndrome as a rare liver disorder is approximately 1:30,000-1:50,000. Its major clinical symptoms include ophthalmologic peculiarity, external phenotype, and congenital defects. The implementation of genetic test information provides next-generation sorting to discover small deletion-insertion, single nucleotide, and variant genes, driving the alagille syndrome market.
Natural therapies are considered to be typical and conventional care for rare inborn disorders by 2036. For instance, CRISPR-Cas9 is a gene-based editing procedure that is likely to successfully fix or replace faulty genes in the upcoming years as reported by NLM in December 2021. This factor is constituted to positively impact the market within the forecast period. Besides, strategies such as reduction in congenital irregularities, occurrence of inborn diseases, native illnesses, and provision of information regarding the availability and implications of carrier evaluation of rare diseases are driving factors of the market.
Key Alagille Syndrome Market Insights Summary:
Regional Highlights:
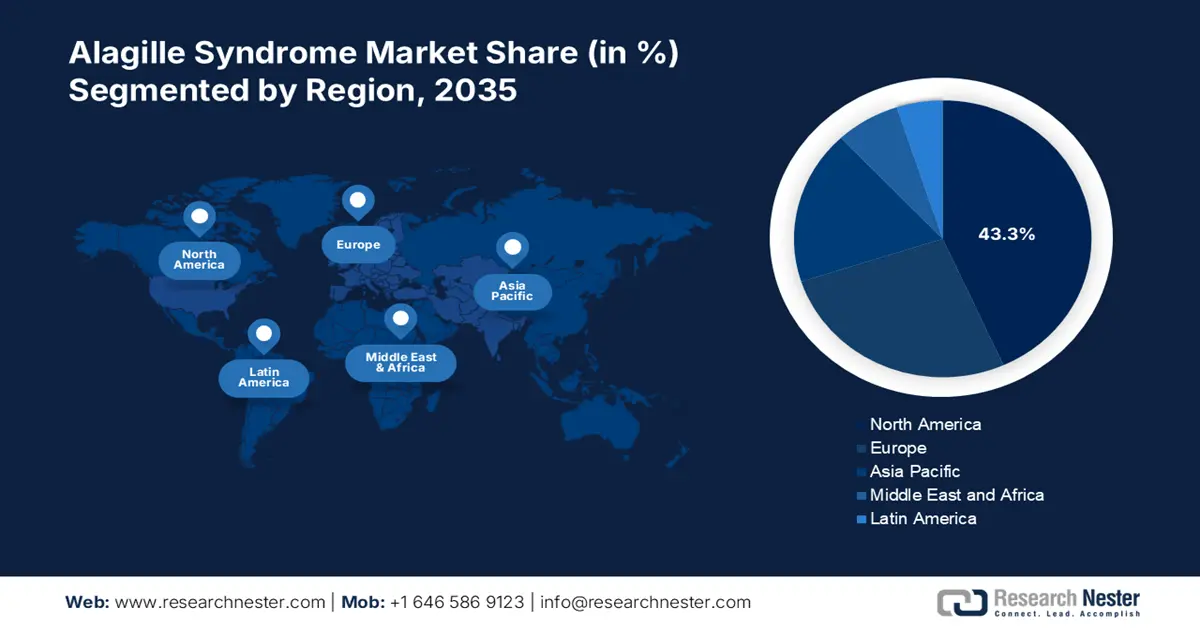
- North America dominates the Alagille Syndrome Market with a 43.3% share, driven by increased diagnosis and treatment advancements, along with rising awareness of Alagille syndrome, fostering robust growth through 2026–2035.
- The Asia Pacific Alagille Syndrome Market is poised for substantial growth by 2035, driven by rising genetic prevalence, enhanced diagnosis, and high number of clinical trials in countries like Japan, China, and Australia.
Segment Insights:
- The Ursodeoxycholic Acid segment is forecasted to achieve a 39.80% market share by 2035, propelled by its use as a first-line therapy for children.
- The Hospital Pharmacies segment is projected to grow at a considerable rate in the Alagille Syndrome Market from 2026-2035, fueled by enhanced pharmaceutical care post-COVID-19.
Key Growth Trends:
- Rising incidence of cancer
- Increasing demand for novel therapies
Major Challenges:
- Limited awareness of alagille syndrome
- High treatment cost
- Key Players: Teva Pharmaceutical Industries Ltd., Shire, Lannett, Novartis International AG, Mylan N.V..
Global Alagille Syndrome Market Forecast and Regional Outlook:
Market Size & Growth Projections:
- 2025 Market Size: USD 430.43 million
- 2026 Market Size: USD 453.29 million
- Projected Market Size: USD 763.59 million by 2035
- Growth Forecasts: 5.9% CAGR (2026-2035)
Key Regional Dynamics:
- Largest Region: North America (43.3% Share by 2035)
- Fastest Growing Region: Asia Pacific
- Dominating Countries: United States, Germany, United Kingdom, France, Japan
- Emerging Countries: China, India, Japan, South Korea, Singapore
Last updated on : 13 August, 2025
Alagille Syndrome Market Growth Drivers and Challenges:
Growth Drivers
- Rising incidence of cancer: Patients with alagille syndrome constitute an increased risk of cancer, especially hepatocellular carcinoma (HCC) owing to chronic liver disorder. Besides, 77% increase in cancer cases has been reported by the World Health Organization 2024 globally since the estimated 20 million cases registered in 2022. By 2050, it is expected that there will be 35 million new cases prevailing worldwide. However, to keep a check on this, the National Policy for Rare Diseases 2021 funded USD 5 million to every patient, despite their economic status, as reported by the Organization for Rare Diseases of India. For instance, there are 11 Centres of Excellence in Karnataka and a one-time grant of USD 50 million has been allocated to establish this. This is the first state in the country to launch a Centre of Excellence for Rare Disorders, located within the Indira Gandhi Institute of Child Health, and to initiate treatment. Hence, such initiatives lead to the advancement of the alagille syndrome market to combat rare diseases.
- Increasing demand for novel therapies: The presence of JAG1 and NOTCH2 genes leading to alagille syndrome requires the demand for novel therapy treatments. Additionally, pulmonary stenosis is common with alagille, resulting in cardiac arrythmias among 63% to 98% of patients as reported by NLM in August 2023. At present, the Ileal Bile Acid Transporter (IBAT) is an appropriate therapeutic approach that restricts enterohepatic circulation and the Partial External Biliary Diversion (PEBD) is a highly invasive procedure. Both these therapies help in overcoming the syndrome, thus a driving factor to enhance the alagille syndrome market.
Challenges
- Limited awareness of alagille syndrome: It is a rare liver disorder and the majority of patients ignore symptoms associated with it due to which there is less awareness. This eventually results in delayed treatment and diagnosis which later on caters to significant consequences such as liver failure, heart disorders, and stroke owing to blood vessel issues. Raising awareness about this syndrome by educating people globally to identify symptoms and ensure suitable treatment is a necessity. For instance, to create awareness regarding the syndrome, the Alagille Syndrome Alliance (ALGSA) provides exclusive collection of merchandise and clothing, which can uplift the alagille syndrome market.
- High treatment cost: There is a variety of treatment options available to combat alagille syndrome and the cost differs from each and every treatment. These poses a hefty restraint, especially in low-income or developing nations and the availability of these treatments is limited. Hence, patients with high-cost burdens are unable to undertake medical decisions and constitute a negative mindset, thereby impacting the alagille syndrome market.
Type-based cost analysis
|
Treatment Type |
Approximate Cost |
|
Ursodeoxycholic Acid |
USD 30 to 60 |
|
Vitamin Supplementation (A, D, E, K) |
USD 10 to 50 |
|
Cholestyramine |
USD 20 to 100 |
|
Livmarli |
USD 2,500 to 4,500 |
|
External Biliary Diversion |
USD 20,000 to 50,000 |
|
Liver Transplant |
USD 100,000 to 500,000 |
|
Experiment medication |
Varies |
Source: every one.org 2024
Alagille Syndrome Market Size and Forecast:
| Report Attribute | Details |
|---|---|
|
Base Year |
2025 |
|
Forecast Period |
2026-2035 |
|
CAGR |
5.9% |
|
Base Year Market Size (2025) |
USD 430.43 million |
|
Forecast Year Market Size (2035) |
USD 763.59 million |
|
Regional Scope |
|
Alagille Syndrome Market Segmentation:
By Drug (Ursodeoxycholic Acid, Cholestyramine, Rifampicin, Late Stage Pipeline Drugs)
Ursodeoxycholic acid segment is anticipated to capture over 39.8% alagille syndrome market share by 2035, based on the drug class. Ursodeoxycholic acid is a first-line therapy, especially for children with the syndrome that stimulates the bile flow. Besides, the Egyptain Liver Journal in 2023 stated that 10% to 15% of patients with alagille syndrome face neonatal cholestasis with an absence of gender predominance. The journal conducted a study wherein an 18-year-old male patient was diagnosed with bile duct paucity at birth. As a treatment, the patient was provided with a vitamin K supplement along with propranolol and ursodeoxycholic acid for the liver, a hypercaloric diet for malnutrition, and other therapeutic attention. After a year of treatment, he displayed improvement in health, thereby showcasing the need for ursodeoxycholic acid to uplift the market.
By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies)
The hospital pharmacies segment is expected to influence the alagille syndrome market at a considerable rate by the end of 2035. There has been an enhancement in pharmacies since the onset of COVID-19 pandemic. Hospital pharmacies play a pivotal role in the evolution and distribution of drugs to ensure effective care for patients. The NLM in October 2024 stated that in Poland, both prescribed and over-the-counter medicines are purchased from pharmacies. At times, due to the aging aspect, medical necessity exceeds leading to polypharmacy. Mostly 50% of elder patients purchase over five medicines which affect their health resulting in contraindicated combinations and dual therapy requirements, impacting the alagille syndrome market growth.
Our in-depth analysis of the global market includes the following segments:
|
By Drugs |
|
|
By Distribution Channel |
|

Vishnu Nair
Head - Global Business DevelopmentCustomize this report to your requirements — connect with our consultant for personalized insights and options.
Alagille Syndrome Market Regional Analysis:
North America Market Analysis
North America alagille syndrome market is set to account for revenue share of more than 43.3% by the end of 2035. According to the American Liver Foundation 2025, the prevalence of alagille syndrome is 1 in every 30,000 live births in America and the disorder equally impacts both males and females, displaying no racial, ethnic, or geographical preferences. Moreover, features that are essential to implement diagnosis include abnormalities in the spine, eyes, cardiovascular system, and kidneys. Additionally, 8.4% of the population over 40 years of age in North America suffer from farsightedness and 23.9% catre to nearsightedness as a unique abnormality in their eyes, as stated by NVISION in December 2024.
The U.S. alagille syndrome market is gaining traction due to the challenges of diagnosing the syndrome. This is due to clinical manifestations that either lead to the absence of symptoms to life-threatening situations. However, the U.S. Johns Hopkins Medicine 2025 has come up with specific treatments to aid the alagille syndrome, including medication like theophylline, insulin glucagon, and phenobarbitone to increase the bile flow, utilization of nonprescription corticosteroid cream to reduce itching, intake of vitamin and high-calorie supplements, and liver transplantation in case there is liver failure. All these treatments are effectively navigating the thriving market.
The alagille syndrome market in Canada is witnessing significant growth due to the enhancement in understanding the syndrome. As per the Canadian Liver Foundation 2025, a child has a 50% likelihood of inheriting alagille syndrome if one of the parents is affected by it. The foundation has also ensured that medical treatment is the most suitable ailment for the syndrome. This is further based on enhancing the bile flow from the liver, nurturing the standard growth and development, and preventing nutritional deficiencies. Besides, in November 2023, Medison Pharma and Ipsen declared the Health Canada approval of Bylvay to provide ailment in patients suffering from life-threatening liver disease, a proactive way to positively impact the market.
APAC Market Analysis
The alagille syndrome market in APAC is gaining traction and is poised to witness lucrative growth during the forecast timeline. A rise in genetic prevalence and enhancement in recognizing the syndrome are the main factors driving the market growth in the region. Besides, the Asia Pacific region has been the largest contributor with more than 50% clinical trials in comparison to other global regions, as stated by Novotech in May 2022. As per the WHO report published in December 2024, a substantial number of trials took place in Japan, accounting for 65,167. In Australia and China, the trial numbers were 32,924 and 135,747, thereby positively impacting the market in terms of clinical trials.
The alagille syndrome market in India is expecting substantial growth due to the rise in rare disease prevalence. For instance, Karnataka accounts for 3.5 million rare disease cases which is 6% of the total population in the state, as stated by the Organisation of Rare Diseases of India (ORDI) in May 2023. The incidence rate of the syndrome is 50% among children, resulting in fatalities in different age groups, highly impacting the market upliftment.
Fatality percentage for different age group of children
|
Age |
Fatality Percentage |
|
Before 1 year |
35% |
|
Between 1-5 years |
10% |
|
Between 5-15 years |
12% |
Source: Organisation of Rare Diseases of India (ORDI) 2023
The alagille syndrome market in China is gaining traction owing to clinical penetrance and hereditary variations of children with the syndrome. As per a clinical study conducted by NCBI in November 2022, 10 children from China with alagille syndrome affected their different organs. However, in May 2023, the China Medical System (CMS) stated the NDA of Tildrakizumab Injection, known as ILUMETRI, to provide treatment to adults suffering from moderate-to-severe plaque psoriasis. Since alagille syndrome affects body organs, this particular treatment proved useful to initiate systematic therapy, positively impacting the alagille syndrome market.
Alagille syndrome impact percentage on body organs
|
Organs |
Impact |
|
Liver |
100% |
|
Heart |
70% |
|
Facial features |
70% |
|
Skeletal |
40% |
|
Brain |
10% |
|
Kidney |
30% |
Source: NLM 2022
Key Alagille Syndrome Market Players:
- Teva Pharmaceutical Industries Ltd.
- Company Overview
- Business Strategy
- Key Product Offerings
- Financial Performance
- Key Performance Indicators
- Risk Analysis
- Recent Development
- Regional Presence
- SWOT Analysis
- AbbVie, Inc.
- Glenmark Pharmaceuticals Ltd.
- Mylan Pharmaceuticals, Inc.
- Mylan N.V.
- Albireo Pharma, Inc.
- Akron, Inc.
- Novartis International AG
- Shire
- Lannett
Companies dominating the alagille syndrome market are gaining rapid exposure due to the escalation in clinical trials combined with a rise in funding and investment for the treatment of alagille syndrome. Additionally, key players are focused on the evolution of the latest drugs for providing treatment to cure the syndrome globally. Sponsorship for the establishment of healthcare facilities is yet another factor driving the influence of key players for market development.
In March 2021, RiverVest Venture Partners shut down its Venture Fund V, only to focus on making investments worth USD 275 million in medical devices and early-stage biopharma companies. The objective was to initiate expenditure in collaboration with academic investigators and entrepreneurs to introduce products for challenges faced by patients, especially with rare diseases. This denotes a positive impact on the future upliftment of the alagille syndrome market in terms of getting funding for the latest drug development.
Recent Developments
- In September 2024, Ipsen declared the approval of Kayfanda by the European Commission to provide treatment of cholestatic pruritus in alagille syndrome for patients between the age range of 6 months and older.
- In May 2024, CANbridge Pharmaceuticals, Inc. stated that LIVMARLI gained expansion by the National Medical Products Administration (NMPA) to combat alagille syndrome among patients aged three months and older.
- Report ID: 7089
- Published Date: Aug 13, 2025
- Report Format: PDF, PPT
- Explore a preview of key market trends and insights
- Review sample data tables and segment breakdowns
- Experience the quality of our visual data representations
- Evaluate our report structure and research methodology
- Get a glimpse of competitive landscape analysis
- Understand how regional forecasts are presented
- Assess the depth of company profiling and benchmarking
- Preview how actionable insights can support your strategy
Explore real data and analysis
Frequently Asked Questions (FAQ)
Alagille Syndrome Market Report Scope
Free Sample includes current and historical market size, growth trends, regional charts & tables, company profiles, segment-wise forecasts, and more.
Connect with our Expert
Copyright @ 2026 Research Nester. All Rights Reserved.




