Adenovirus Based Virotherapy Market Outlook:
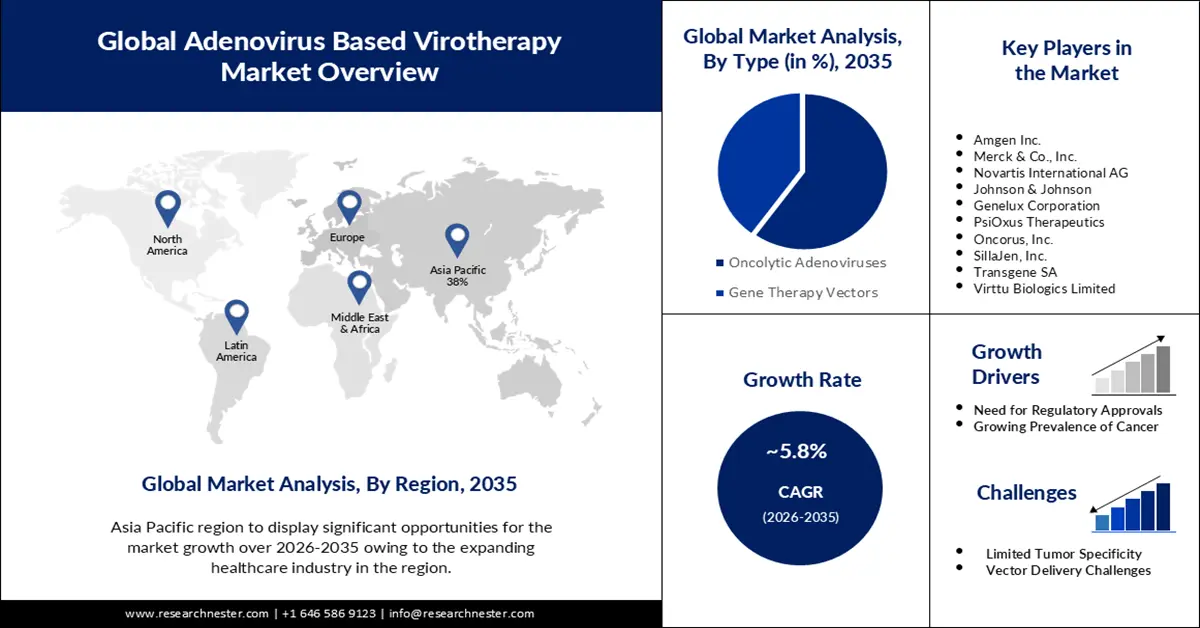
Adenovirus Based Virotherapy Market size was valued at USD 14.5 billion in 2025 and is likely to cross USD 25.48 billion by 2035, expanding at more than 5.8% CAGR during the forecast period i.e., between 2026-2035. In the year 2026, the industry size of adenovirus based virotherapy is estimated at USD 15.26 billion.
The development and growth of the market is fueled by several factors, including ongoing clinical trials, regulatory approvals, and the commercialization of virotherapies. Positive results from clinical trials demonstrating the safety and efficacy of adenovirus-based virotherapy in treating certain types of cancer significantly drive market growth. Successful clinical outcomes contribute to regulatory approvals and increased industry interest. Along with these, escalating research & development concerned with adenovirus and surging demand for vector-based therapies are also projected to drive market growth in the coming years. Furthermore, rising government investments to spread awareness about early cancer detection are expected to provide ample growth opportunities to the market in the near future.
Adenovirus-based virotherapy involves the use of adenoviruses, which are common viruses that can cause respiratory and other infections in humans, as vectors to deliver therapeutic genes into cancer cells. These viruses are modified to selectively replicate in and destroy cancer cells while sparing normal, healthy cells. Obtaining regulatory approvals from agencies such as the FDA or EMA for adenovirus-based virotherapies is a critical factor for market growth. Regulatory clearance enables companies to bring their products to market and increases confidence among healthcare professionals and patients.